��Ŀ����
ij��ѧ�о���ѧϰС���ͬѧ����������Ȥζʵ�飬װ������ͼ�����������ã�����������Һ©����������Һ�����ʢ�й�����Թ�2��ʱ���۲쵽��ͬ��������1�����Թ�2�з����˻�ѧ��Ӧ���۲쵽�Թ�1�������ݲ����������е�ʯ����Һ��죬�Թ�2�з�����Ӧ�Ļ�ѧ����ʽΪ
��2�����Թ�2��û�з�����ѧ��Ӧ���۲쵽��֧���ܿ�ͬʱð���ݡ�����ƿ�ڵ�ʯ��ˮ����ǣ����Թ�2�еĹ��������
��3�����۲쵽�ձ��е�Һ�嵹��������ƿ�ڣ���ʹ����ƿ��ʢ�еĺ�ɫ��Һ����ɫ�����Һ©���е�Һ�塢�Թ�2�еĹ���ֱ���
��4������ʵ���е�ʯ��ˮ�к���һ�ּ�--�������ƣ���������Ҳ�����dz��õ�һ�ּ��ҵ��ͨ���õ���Ȼ�����Һ�ķ�������ȡ����Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O ͨ��Cl2��+H2��+2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ�����ȫ��Ӧʱ���õ�109��7g��Һ������������������ʱ��Ĺ�ϵ����ͼ��ʾ������㣺
���Ȼ�����ȫ��Ӧʱ����Һ��ʣ��ˮ��������
���Ȼ�����Һ�����ʵ�����������
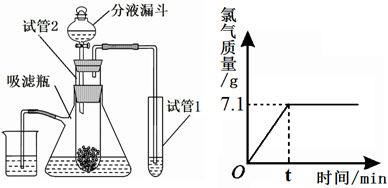
��������ͼ�ɿ������Ȼ�����ȫ��Ӧʱ�ɵõ�����7.1g��
�ٷ�Ӧ������������Һ�ڰ����������ƺ�ˮ����������������������������Ƶ����������÷�Ӧ������������Һ������-�������Ƶ�������Ϊ��Һ��ʣ��ˮ��������
��ԭ�Ȼ�����Һ�����ʵ���������=
��100%���Ȼ��Ƶ������ɸ���������������������������غ㶨�ɣ��Ȼ�����Һ������=����������+�������������ɸ������������������+��Ӧ������������Һ��������
�ٷ�Ӧ������������Һ�ڰ����������ƺ�ˮ����������������������������Ƶ����������÷�Ӧ������������Һ������-�������Ƶ�������Ϊ��Һ��ʣ��ˮ��������
��ԭ�Ȼ�����Һ�����ʵ���������=
| �Ȼ��Ƶ����� |
| �Ȼ�����Һ������ |
����⣺��1��������ʹʯ����Һ��������˵����һ���������壻�ʴ�Ϊ��CaCO3+2HCl�TCaCl2+H2O+CO2��
��2���ܽ��ܷų����������ʣ��ʴ�Ϊ��NaOH
��3���ܽ��ܷų����������ʣ���ɫ��Һ����ɫ˵��������������Һ���ʴ�Ϊ��ˮ������泥���ɱ���������
�Թ�2�еĹ����ܽ⣨����Һ�巴Ӧ��ʱ����������ʹ����ƿ�е�ѹǿ��С���ձ��е��ᵹ������ƿ�У�����ƿ�м������ʷ�����Ӧ��ʹ��ɫ��Һ����ɫ��
��4�����������������Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��������������Ϊz
2NaCl+2H2O�TCl2��+H2��+2NaOH
117 71 2 80
y 7.1g z x
=
=
=
x=8g y=1.7g z=0.2g
ʣ��ˮ��������109.7g-8g=101��7g
���Ȼ�����Һ����Ϊ��109.7g+7��1g+0.2g=117g
�Ȼ�����Һ�����ʵ���������Ϊ��
��100%=10%��
���Ȼ�����Һ�����ʵ���������Ϊ10%
��2���ܽ��ܷų����������ʣ��ʴ�Ϊ��NaOH
��3���ܽ��ܷų����������ʣ���ɫ��Һ����ɫ˵��������������Һ���ʴ�Ϊ��ˮ������泥���ɱ���������
�Թ�2�еĹ����ܽ⣨����Һ�巴Ӧ��ʱ����������ʹ����ƿ�е�ѹǿ��С���ձ��е��ᵹ������ƿ�У�����ƿ�м������ʷ�����Ӧ��ʹ��ɫ��Һ����ɫ��
��4�����������������Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��������������Ϊz
2NaCl+2H2O�TCl2��+H2��+2NaOH
117 71 2 80
y 7.1g z x
| 71 |
| 7.1g |
| 80 |
| x |
| 71 |
| 7.1g |
| 117 |
| y |
| 71 |
| 7.1g |
| 2 |
| z |
x=8g y=1.7g z=0.2g
ʣ��ˮ��������109.7g-8g=101��7g
���Ȼ�����Һ����Ϊ��109.7g+7��1g+0.2g=117g
�Ȼ�����Һ�����ʵ���������Ϊ��
| 11.7g |
| 117g |
���Ȼ�����Һ�����ʵ���������Ϊ10%
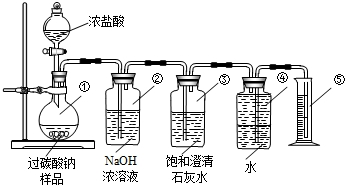
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�
ע�⣺���㷴Ӧ����Һ����ʱ���÷�Ӧǰ��Һ������ȥ�������������ʱ������Ҫ��ȥ��������������Ҫ��ȥ������������
ע�⣺���㷴Ӧ����Һ����ʱ���÷�Ӧǰ��Һ������ȥ�������������ʱ������Ҫ��ȥ��������������Ҫ��ȥ������������

��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
�����Ŀ
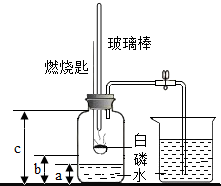 ij��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ��ʵ��Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ���ͼ��ʾ��ʵ��װ�ã�
ij��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ��ʵ��Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ���ͼ��ʾ��ʵ��װ�ã�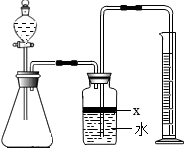 ��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮
��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮