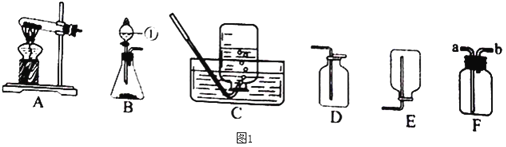
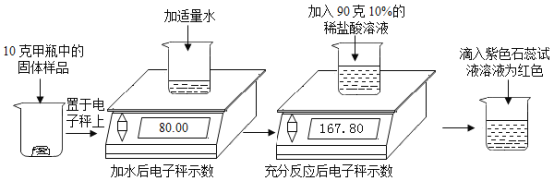
��Ŀ����
����Ŀ���ϳ����ǹ�ҵ�����е�һ��ԭ��������Ҫ�ɷ���һ����̼��������������ұ�����������������ѵȡ������ͼʾ�ش�

(ע��ͼ�������ڻ�ѧʽ��ʾ��Ӧ���ʵ���Ҫ�ɷ�)
(1)��д�����úϳ��������Ļ�ѧ����ʽ________(дһ������)��
(2)������(CH3OCH3)����Ϊ21��������ȼ�ϣ���ʵ�ָ�Ч���ȼ�գ���д���������ڿ����г��ȼ�����ɶ�����̼��ˮ�Ļ�ѧ����ʽ______��
(3)�ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʡ����úϳ���Ϊԭ�ϲ����ܵõ���������_______(����ĸ���)
A���״�(CH3OH) B���Ҷ�ȩ(HC2O2) C������[CO(NH2)2]
���𰸡� 3CO��Fe2O3![]() 2Fe��3CO2(��3H2��Fe2O3
2Fe��3CO2(��3H2��Fe2O3![]() 2Fe��3H2O) C2H6O��3O2
2Fe��3H2O) C2H6O��3O2![]() 2CO2��3H2O C
2CO2��3H2O C
��������
��1��һ����̼���������л�ԭ���ܶ�ȡ�����������е������������úϳ��������Ļ�ѧ����ʽ�ǣ�3CO��Fe2O3![]() 2Fe��3CO2(��3H2��Fe2O3
2Fe��3CO2(��3H2��Fe2O3![]() 2Fe��3H2O) ��
2Fe��3H2O) ��
��2���������ڿ����г��ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽ�ǣ�C2H6O��3O2![]() 2CO2��3H2O��
2CO2��3H2O��
��3�����������غ㶨�ɿ�֪����ѧ��Ӧǰ��Ԫ�ص�����䣬�ϳ�����CO��H2���ڲ�ͬ�����������£��ϳɵ����ʺ���̼Ԫ�ء���Ԫ�غ���Ԫ�أ������ܺ��е�Ԫ�أ������ܵõ������������ء�