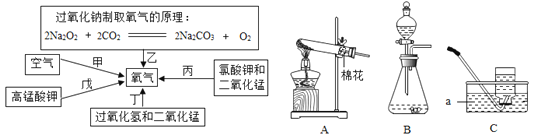
��Ŀ����
����Ŀ��ʵ�����м�����ƿ���õ��������ƹ��壬ijѧϰС��Ϊ���о���������������������ʵ��: (���ӳ�ʾ����λΪ��)
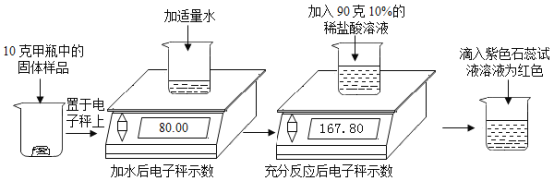
(1)������ɫʯ����Һ����ҺΪ��ɫ��˵����Ӧ����Һ��____�ԡ�
(2)����ʵ���в����Ķ�����̼��������Ϊ______�ˡ�
(3)�����ƿ������Ʒ��̼���Ƶ���������____________��
(4)ijͬѧ��ȡ10����ƿ�еĹ�����Ʒ,��100��15%��ϡ���ᰴͬ����������ʵ�飬����Ϊ���ܹ�����Ʒ���ʳ̶���Σ�ϡ������������Ҫʹ��ʯ����Һ�������˵�����������жϵ�ԭ��__________��
���𰸡� �� 2.2 53% ��100g15%��ϡ�������ʵ�飬ϡ����һ����������ϡ����������Ҫ��ʯ����Һ��
�����������⿼���˸��ݻ�ѧ����ʽ���м��㡣���������غ�����õ�������̼�����������ݶ�����̼��������ϻ�ѧ����ʽ������Ʒ��̼���Ƶ���������һ�����������Ʒ��̼���Ƶ�����������
��1����ɫʯ����Һ��������Һ���ɫ��������ɫʯ����Һ����ҺΪ��ɫ��˵����Ӧ����Һ�����ԡ�
��2�����������غ㣬�����Ķ�����̼��������Ϊ80.00g+90g-167.80g=2.2g��
��3���������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
![]() x=5.3g
x=5.3g
������Ʒ��̼���Ƶ���������=![]() ��100%=53%��
��100%=53%��
��4������Ʒ��ȫ���ʣ���Ʒȫ�����̼���ơ�
�裺��10g̼���Ʒ�Ӧ�����������Ϊy��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 98
10g y
![]() y=9.25g������ϡ���������=
y=9.25g������ϡ���������=![]() =61.64g��61.64g<100g��ϡ���������
=61.64g��61.64g<100g��ϡ���������
����Ʒû�б��ʣ���Ʒ����10g���������ơ�
�裺��10g�������Ʒ�Ӧ�����������Ϊz��
2NaOH+H2SO4=Na2SO4+2H2O
80 98
10g z
![]() z=12.25g������ϡ���������=
z=12.25g������ϡ���������=![]() =81.67g��81.67g<100g��ϡ���������
=81.67g��81.67g<100g��ϡ���������
���Բ��ܹ�����Ʒ���ʳ̶���Σ�����100g15%ϡ�����ϡ����һ�����������������жϵ�ԭ������100g15%��ϡ�������ʵ�飬ϡ����һ����������ϡ����������Ҫ��ʯ����Һ��