��Ŀ����
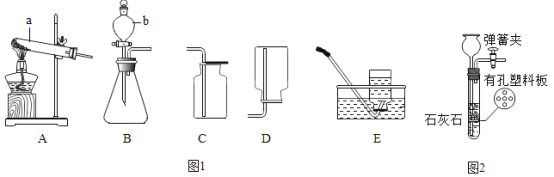
����Ŀ����6�֣�ijȼ��X��7.2g������ȼ�գ��õ�9.2g������壨���ܺ���һ����̼��������̼��ˮ����������ѧ��ȤС���ͬѧ������ͼ��ʾװ����֤�������ijɷ֡�
���ϣ���Ũ���������ˮ�ԡ���Ũ����������Һ�����ն�����̼��
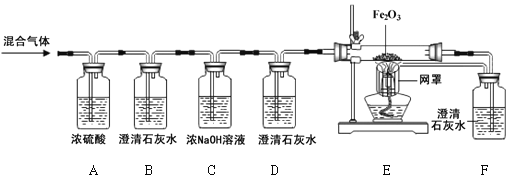
��1��ͨ����������Aװ������4.5g��˵����������к���________________________��
��2��Bװ���г���ʯ��ˮ����ǣ�������Ӧ�Ļ�ѧ����ʽΪ ��Dװ�õ������� ��
��3�����۲쵽 ��֤�������������CO��
��4��ʵ�������ͬѧ�dz���B��Cװ�ù�����3.3g�������������ݿ�ȷ��ȼ��X������Ԫ�ؼ�Ԫ��������Ϊ_________________��
���𰸡���1��ˮ����
��2��CO2+Ca��OH��2 === CaCO3��+H2O ���������̼�Ƿ����
��3��E�к�ɫ�����ڣ�F��ʯ��ˮ����ǣ�
��4��C��H = 3:1
��������
���������װ����A����ˮ�����ã��ɸ���Ũ�������ӵ�������ˮ����������������Aװ������4.5g��˵����������к���ˮ����4.5g�������ʯ��ˮ������������̼�Ĵ��ں����ն�����̼�����ã������ж�����̼����ôB�е�ʯ��ˮ�����ǣ�CO2+Ca��OH��2 === CaCO3��+H2O��C�û��е��������������ն�����̼��D�Ǽ��������̼�Ƿ�������������ʵ�飻Eװ���Ǽ���һ����̼�Ĵ��ڣ�������һ����̼����ôһ����̼������������Ӧ���ɺ�ɫ��������F�еij���ʯ��ˮ��������B��Cװ�������ӵ�������Ϊ��������ж�����̼���������ʻ�������ж�����̼������Ϊ3.3g����ôˮ�����Ͷ�����̼��������Ϊ4.5g+3.3g=7.8g�����������غ㶨�ɻ�ѧ��Ӧǰ�����ʵ����������䣬��������������Ϊ9.2g���ʻ��������һ����̼������Ϊ9.2g-7.8g=1.4g�����������غ㶨�ɻ�ѧ��Ӧǰ��Ԫ�ص����������䣬�μӷ�Ӧ������������Ϊ7.2g����ôX������Ϊ2g��ˮ����ԭ�ӵ�����Ϊ4.5g��2��18 ��100% =0.5g����Ԫ�ص�����=4.5g-0.5g=4g��������̼��̼Ԫ�ص�����Ϊ3.3g��12��44 ��100%=0.9g����Ԫ�ص�����=3.3g-0.9g=2.4g��һ����̼��̼Ԫ�ص�����Ϊ1.4g��12��28 ��100%=0.6g����Ԫ�ص�����=1.4g-0.6g=0.8g,���������Ԫ�ص�����=4g+2.4g+0.8g=7.2g����X�к���̼��������Ԫ�أ�̼Ԫ�ص�����=0.9g+0.6g=1.5g����Ԫ�ص�����=0.5g����ȼ��X������Ԫ�ص�������ΪC��H = 3:1��