��Ŀ����
�ҹ�ij����ҵ������еIJ��ֵ���������ˮƽ��pH���±�����ش��������⣺
��1��������ˮ��pHԼΪ5.6�����û�ѧ����ʽ��ʾ��ԭ��Ϊ �������pHС��5.6�����ϱ��оٵ���������У�û��������Ⱦ�ĵ����� ��
��2�����е����������Ҫ������ ������ţ�����CO����CO2����SO2����NO2��
��3��Ϊ�˸��Ʊ��л��������������������������д�ʩ���ܼ���������� ������ţ���
���ƹ������Դ���ڼ�����úֱ����ȼ�ϣ�����̭β��������������������ȼ���̻��������Ʋ��г�ʹ�����ϴ���
| �������� | A�� | B�� | C�� | D�� | E�� |
| �ⶨ��ˮpH��ƽ��ֵ | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 |
��2�����е����������Ҫ������
��3��Ϊ�˸��Ʊ��л��������������������������д�ʩ���ܼ����������
���ƹ������Դ���ڼ�����úֱ����ȼ�ϣ�����̭β��������������������ȼ���̻��������Ʋ��г�ʹ�����ϴ���
���㣺����IJ�����Σ��������,��Һ���������pHֵ�Ĺ�ϵ,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧ�뻷������
��������1�����ݿ����еĶ�����̼��ˮ��Ӧ����̼���𣻸��ݱ�����Ϣ���
��2������ˮ��pH��5.6ʱ��Ϊ���꣬�����������������γ��������Ҫ���ʣ�
��3�����Ʋ��г�ʹ�����ϴ����ܼ�������IJ�����
��2������ˮ��pH��5.6ʱ��Ϊ���꣬�����������������γ��������Ҫ���ʣ�
��3�����Ʋ��г�ʹ�����ϴ����ܼ�������IJ�����
����⣺
��1�������еĶ�����̼��ˮ��Ӧ����̼�ᣬ���������ˮ��pHԼΪ5.6����ѧ����ʽ��ʾ��ԭ��ΪCO2+H2O=H2CO3��D������ˮ��pH��6.37������5.6��û���ܵ�������Ⱦ��
��2�������������������γ��������Ҫ���ʣ�
�ʴ�Ϊ����Դ���ۢܣ�
��2���ƹ������Դ��������úֱ����ȼ�ϡ���̭β������������������ȼ���̻�����ȴ�ʩ�ܼ�������IJ��������Ʋ��г�ʹ�����ϴ����ܼ�������IJ�����
�𰸣�
��1��CO2+H2O=H2CO3��D������2���ۢܣ���3���ݣ�
��1�������еĶ�����̼��ˮ��Ӧ����̼�ᣬ���������ˮ��pHԼΪ5.6����ѧ����ʽ��ʾ��ԭ��ΪCO2+H2O=H2CO3��D������ˮ��pH��6.37������5.6��û���ܵ�������Ⱦ��
��2�������������������γ��������Ҫ���ʣ�
�ʴ�Ϊ����Դ���ۢܣ�
��2���ƹ������Դ��������úֱ����ȼ�ϡ���̭β������������������ȼ���̻�����ȴ�ʩ�ܼ�������IJ��������Ʋ��г�ʹ�����ϴ����ܼ�������IJ�����
�𰸣�
��1��CO2+H2O=H2CO3��D������2���ۢܣ���3���ݣ�
�����������Ĺؼ�����������������γɡ���ֹ������Ⱦ�ķ����ȷ����֪ʶ��ֻ���������ܶ�����������ȷ���жϣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
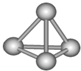 �����������ѧ�Ŀ�ѧ�һ���˼��������о������N4���ӣ�N4���ӽṹ��ͼ��ʾ�������й�N4��˵����ȷ���ǣ�������
�����������ѧ�Ŀ�ѧ�һ���˼��������о������N4���ӣ�N4���ӽṹ��ͼ��ʾ�������й�N4��˵����ȷ���ǣ�������| A��N4��һ�ֻ���� |
| B��N4��һ�ֵ��� |
| C��N4����һ�����͵Ļ����� |
| D������N4��N2�����Ԫ����ͬ���������ǵ�������ͬ |
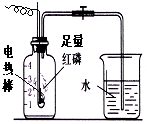 ����ͼ��װ�������ر�ֹˮ�У�ͨ��ʹ����ȼ�գ���ش��������⣺
����ͼ��װ�������ر�ֹˮ�У�ͨ��ʹ����ȼ�գ���ش��������⣺ ͼ��������ʾij���ʷ�����ѧ�仯����ʾ��ͼ��ͼ��
ͼ��������ʾij���ʷ�����ѧ�仯����ʾ��ͼ��ͼ��  ��
�� �ֱ��ʾ����Ԫ�ص�ԭ�ӣ���ϸ�۲�ͼ���ش��������⣺
�ֱ��ʾ����Ԫ�ص�ԭ�ӣ���ϸ�۲�ͼ���ش��������⣺