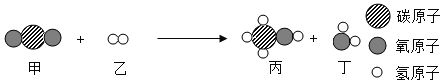
��Ŀ����
����Ŀ��ijУ��ѧ��ȤС����ʵ��������һ��Ũ�ȵ�NaOH��Һ�Ա�ʹ�á�
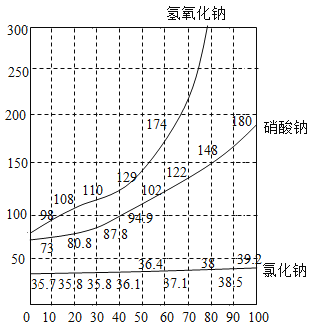
(1)����100gNaOH�ı�����Һ�������õ�����ԼΪ20�棬�����ܽ������ͼ��
��������������Ƶ�����ԼΪ_____g(������С��)��������ˮ�����Ϊ_____mL��(�ܶ�ԼΪ1g/mL)��
������ʵ������������_____��
A ��ƽ��ƽǰ�������Ϸֱ��������ȵ�ֽ
B ����ʱ����ƽָ��ƫ���ƶ���������ƽǡ��ƽ��
C ȷ�����õ��������ƹ������װ��ˮ����Ͳ���ܽ�
D ��Ͳ����ʱ��������Һ�尼Һ����ʹ�����ˮƽ
(2)���������ƺõ���Һ����ϡ����10%���������պ���̽��ʵ�顣ʵ��С��ͬѧȡ��������������Һ10mL���Թ��У���ε���ϡ���ᣬʵ������У����Ǿ���ķ��������ݲ�����ʵ��С���Ʋ⣬����ϡ�����������ݵ�ԭ����_____��
���𰸡�52 48 ABC �������Ʊ������ɵ�̼���ƺ�ϡ���ᷴӦ
��������
��1����20��ʱ��NaOH�ܽ��Ϊ109g�����¶��£�������Һ��������������Ϊ��![]() ��������������Ƶ�����ԼΪ100g��52%��52g��������ˮ������Ϊ100g-52g��48g������Ҫˮ�����Ϊ48mL�����52��48��
��������������Ƶ�����ԼΪ100g��52%��52g��������ˮ������Ϊ100g-52g��48g������Ҫˮ�����Ϊ48mL�����52��48��
��A���������Ƹ�ʴ�Խ�ǿ�����ܷ���ֽ�ϳ�����Ӧ���ڲ������������ձ����н��г�������ѡ��˵������
B������ʱ������ƽָ��ƫ��˵��ҩƷ������������������Ӧ����ҩƷ����ѡ��˵������
C���ܽ������������Ͳ�ڽ��У�Ӧ���ձ��н��У���ѡ��˵������
D������Ͳ��ȡҺ�壬����ʱ��������Һ�尼Һ����ʹ�����ˮƽ����ѡ��˵����ȷ��
��ѡ��ABC��
��2��������������������еĶ�����̼��Ӧ����̼���ƺ�ˮ������ϡ���ᣬ̼���ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���������ݣ�����������Ʊ������ɵ�̼���ƺ�ϡ���ᷴӦ��

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�