��Ŀ����
����Ŀ������̼�����һ����Ҫ����������Ʒ����ͼ��ij����������̼��Ƶ�����������
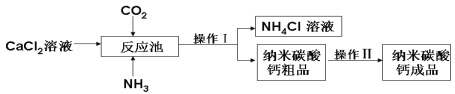
��ش��������⣺
��1��д����Ӧ���з����Ļ�ѧ��Ӧ�Ļ�ѧ����ʽ�� ��
��2����Ӧ����Ҫ��ͨ�백������ͨ�������̼��������Ϊ ��
��3������ʵ�����н��и�ʵ�飬����l�������� ��ʹ�õ��IJ��������в�������___________��____________�����в������������� ��
��4������2Ϊϴ�ӡ� ������ϴ�ӵ�Ŀ���dz�ȥ̼��ƴ�Ʒ���溬�еĿ��������ʣ�����һ�����еĿ����������� �������Ƿ�ϴ�Ӹɾ��ķ����������һ��ϴ��Һ�м��� ��Һ����Ca(OH)2 ���� AgNO3���� CaCl2����д��ţ������û�г��ֳ�����˵���Ѿ�ϴ�Ӹɾ���
��5���±�ΪNH4Cl��ˮ�е��ܽ���б���Ϊ��ø���Ʒ��NH4Cl����Ӧ���� �ᾧ��
T/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
S/g | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 | 65.6 | 71.3 | 77.3 |
���𰸡���1��CaCl2+CO2+2NH3+H2O==2NH4Cl+CaCO3��
��2�������ܽ���ǿ�ڶ�����̼�������ڶ�����̼������
��3������ �ձ� ©�� ����
��4�����ɣ����ɵȣ�NH4Cl�ڣ�5������
��������
�����������Ӧ���з����Ļ�ѧ��Ӧ�Ļ�ѧ����ʽCaCl2 + CO2 + 2NH3 +H2O ==2NH4Cl + CaCO3������Ӧ����Ҫ��ͨ�백������ͨ�������̼��������Ϊ�����ܽ���ǿ�ڶ�����̼�������ڶ�����̼������������ʵ�����н��и�ʵ�飬����l�����������ˣ���Ϊ�����еõ��˹����Һ��ķ�����ʹ�õ��IJ��������в��������ձ� ©�������в�����������������������2Ϊϴ�ӡ����������ϴ�ӵ�Ŀ���dz�ȥ̼��ƴ�Ʒ���溬�еĿ��������ʣ�����һ�����еĿ�����������NH4Cl�������Ƿ�ϴ�Ӹɾ��ķ����������һ��ϴ��Һ�м���AgNO3��Һ�����û�г��ֳ�����˵���Ѿ�ϴ�Ӹɾ��� ����NH4Cl��ˮ�е��ܽ���б������������ʵ��ܽ�����¶ȵĸı�ϴ�Ϊ��ø���Ʒ��NH4Cl����Ӧ�������¡�