��Ŀ����
����ʵ������ȡ����IJ���װ�ã�������ѧ��ѧ֪ʶ���ش��й����⣺


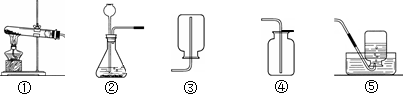
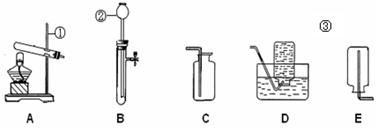
A B C D E
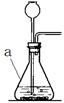
��1��ͼ������a��������________��
��2��ʵ�����ø��������ȡ������װ��A��������һ��Ķ���_________________________��
��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
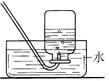
��3��ʵ������ȡ������̼�ķ���װ�ú��ռ�װ����________������ţ�����Ҫ�ռ���һƿ��
��Ķ�����̼���壬Ӧ������װ����Fװ�õ�________���m����n����������
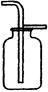
��4������Dװ�õ�������ƿ�����£����ռ���������______________���ξ�һ������





A B C D E
��1��ͼ������a��������________��
��2��ʵ�����ø��������ȡ������װ��A��������һ��Ķ���_________________________��
��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
��3��ʵ������ȡ������̼�ķ���װ�ú��ռ�װ����________������ţ�����Ҫ�ռ���һƿ��
��Ķ�����̼���壬Ӧ������װ����Fװ�õ�________���m����n����������
��4������Dװ�õ�������ƿ�����£����ռ���������______________���ξ�һ������
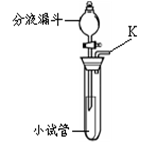
�� ��ƿ
�Թܿڷ���һС���� 2KMnO4��K2MnO4+MnO2+O2��
BD m
�� ����������飬�������ɣ�
�Թܿڷ���һС���� 2KMnO4��K2MnO4+MnO2+O2��
BD m
�� ����������飬�������ɣ�
�����������2���ø��������ȡ�������Թܿ�Ҫ����һС������Ŀ���Ƿ�ֹ������ؽ��뵼��ʹ���ܶ��������ȸ�����طֽ���ȡ������ͬʱ��������������غͶ������̣��ʷ�Ӧ�Ļ�ѧ����ʽ��2KMnO4��K2MnO4 +MnO2 + O2����
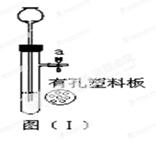
��3�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã���ʯ��ʯ��ϡ����Ϊԭ����ȡ������̼�����ڹ�Һ�峣���µķ�Ӧ����ѡB��Ϊ����װ�ã�������̼�ܶȱȿ�������������ˮ��ֻ���������ſ������ռ���ѡDװ�ã�Ҫ�ռ���һƿ����Ķ�����̼���壬��ôҪ���Ӹ���װ�ã�Ӧ������װ����Fװ�õ�m������
��4����Dװ�õ�������ƿ�����£�����ô���õ��������ſ������ռ����壬�����ռ��ܶȱȿ���С�����壬��������

��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д�
�����Ŀ

 2NaCl������
2NaCl������