��Ŀ����
��֪̼�����Ʋ��ȶ��������ֽ�(2NaHCO3����Na2CO3+H2O+CO2��)����ȡNa2CO3��NaHCO3�Ļ����40g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ27.6 g������˵����ȷ����
A����Ӧ������CO2������Ϊ12.4 g
B����Ӧ��CO2��H2O��������Ϊ22��9
C��ԭ�������NaHCO3������Ϊ16.8 g
D��ԭ�������Na2CO3����������Ϊ16%
�𰸣�BD

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�
�����Ŀ
��֪̼�����Ʋ��ȶ��������ֽ⣨2NaHCO3
Na2CO3+H2O+CO2��������ȡNa2CO3��NaHCO3�Ļ����40g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ27.6g������˵����ȷ���ǣ�������
| ||
| A����Ӧ������CO2������Ϊ12.4g |
| B����Ӧ��CO2��H2O��������Ϊ22��9 |
| C��ԭ�������NaHCO3������Ϊ16.8g |
| D��ԭ�������Na2CO3����������Ϊ16% |
 Na2CO3+CO2��+H2O����ȡNa2CO3��NaHCO3�Ļ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ���ǣ� ��
Na2CO3+CO2��+H2O����ȡNa2CO3��NaHCO3�Ļ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ���ǣ� �� Na2CO3+CO2��+H2O����ȡNa2CO3��NaHCO3�Ļ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ���ǣ� ��
Na2CO3+CO2��+H2O����ȡNa2CO3��NaHCO3�Ļ����10g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ6.9g������˵����ȷ���ǣ� ��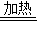 Na2CO3+H2O+CO2��������ȡNa2CO3��NaHCO3�Ļ����40g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ27.6g������˵����ȷ���ǣ� ��
Na2CO3+H2O+CO2��������ȡNa2CO3��NaHCO3�Ļ����40g�����ȵ��������ٸı�Ϊֹ��ʣ���������Ϊ27.6g������˵����ȷ���ǣ� ��