��Ŀ����
����Ŀ����Ƕ���ʶ���ʵı仯�����������Ǹ��õ����⻯ѧ֪ʶ��
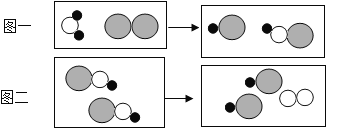
��1�����۽Ƕȣ�ͼ1Ϊij��ѧ��Ӧ����ʾ��ͼ����ͼ�ش��������⡣��
�����Ͽ����û�ѧ�仯�з��������ı������______������ԭ�������������������仯�����У�ͬ��ԭ�ӵĸ���______��������������û������������
���μӷ�Ӧ��A2��B2�������ʵķ��Ӹ�����Ϊ______��
��2���ӷ�Ӧ���ͽǶ�
��һ�����ʿ�ͨ����ͬ�ķ�Ӧ�������ɣ��Զ�����̼Ϊ������
�����������������ɶ�����̼��������______����һ�����ʻ�ѧʽ����
�������£�ʯ��ʯ�ֽ����ɶ�����̼�Ļ�ѧ����ʽ��______��
����һ�������£�̼�������������ܷ����û���Ӧ�����ɶ�����̼��______���ѧʽ����
��3���������仯�Ƕ�
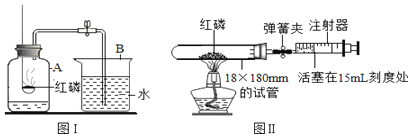
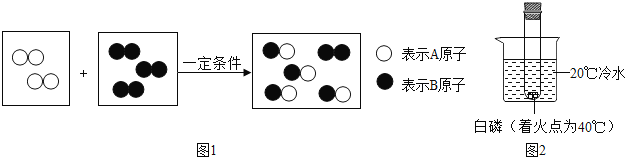
������ͼ2װ�÷ֱ��������ʵ�飬��ʶ���ʱ仯�е������仯��
�����ձ��м���һ������ʯ�һ��������ȼ�գ���ȼ��������������ʱ��ʯ�ҵ�������______��д����ʯ����ˮ��Ӧ�Ļ�ѧ����ʽ______��
����һ����������ij���������ձ��е���ˮ���������������ȼ�յ���______ �����ţ���
A �Ȼ��� B ����� C Ũ���� D ��������
���𰸡����� û�� 1��1 C��CO CaCO3![]() CaO+CO2�� Fe ��ʯ����ˮ��Ӧ�ų��������ȣ�ˮ�����ߣ�ʹ�¶ȴﵽ�˰����Ż�� CaO+H2O=Ca��OH��2 CD
CaO+CO2�� Fe ��ʯ����ˮ��Ӧ�ų��������ȣ�ˮ�����ߣ�ʹ�¶ȴﵽ�˰����Ż�� CaO+H2O=Ca��OH��2 CD
��������
��1�������Ͽ����û�ѧ�仯�з��������ı�����Ƿ��ӣ��仯�����У�ͬ��ԭ�ӵĸ���û��������
�������ı仯��֪����һ��B2����δ�μӷ�Ӧ���μӷ�Ӧ��A2��B2�������ʵķ��Ӹ�����Ϊ 1��1��
��2�������������������ɶ�����̼��������̼��һ����̼����ѧʽ�ǣ�C��CO��
�ڸ����£�ʯ��ʯ�ֽ����ɶ�����̼�������ƣ���ѧ����ʽ�ǣ�CaCO3![]() CaO+CO2����
CaO+CO2����
����һ�������£�̼�������������ܷ����û���Ӧ�����ɶ�����̼������
��3������ʯ����ˮ��Ӧ�ų��������ȣ�ˮ�����ߣ�ʹ�¶ȴﵽ�˰����Ż�㡣��ʯ����ˮ��Ӧ�Ļ�ѧ����ʽ�ǣ�CaO+H2O�TCa��OH��2��
������������ˮʱ�ܷų����������������ȼ�գ�Ũ���ᡢ��������������ˮʱ�ܷų���������ʹ��Һ���¶����ߣ���ѡCD��

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�����Ŀ�������NaCl��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȣ�
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | |
��1����Ҫ�Ƚ�KNO3�� NaCl��ˮ�е��ܽ���������Ҫ���Ƶı�����ˮ��������______��
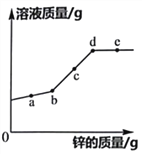
��2��60��ʱ����100 gˮ�м���100 g KNO3���壬��ֽ����õ�______����������������������������Һ����ʱ����Һ�����ʵ���������Ϊ______�������¶���ȴ��20�����ձ����������������Ϊ______g����ʱ��Һ��������������Ϊ______����ȷ��0.01%����
��3����10��ʱ��NaCl������Һ��������������______������������������������С������KNO3������Һ������������������50��ʱ��12gˮ��������ܽ�______ gNaCl��