��Ŀ����
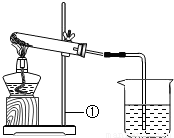
��2011?��������ģ����һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺���ϣ�1���ƾ��Ƽ����¶�Լ500��600�森
2��NaOH 318.4���ۻ����ֽ⣬1390����ڲ��ֽ⣮
3��Na2CO3+CaCl2��CaCO3��+2NaCl��
4��Na2CO3��Һ�ʼ��ԣ�CaCl2��Һ������
��1�����ʵ�鱨������ݣ�
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ ______ ��Ӧ�Ļ�ѧ����ʽ�� ______ |
���𰸡���������1�������Թ��е�������������������Ͻ��з�����
��2����������������Һ�Լ��ԣ���̼����������κ��������ԾͿ��Լ���
����⣺̼���������Ⱥ��ֽ��̼���ơ�ˮ�Ͷ�����̼���������������Ⱥ�ֽ⣬���Ⱥ��Թ��ڱ�����ɫҺ�Σ�������ɫ���壬ʯ��ˮ����ǣ�����һ������̼�����ƣ�
�������ƺ����ᷴӦ�����������壬̼���ƺ����ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼��
��2����������������Ҫ���������Լ��Ե��������κ�����÷�̪���飬
�ʴ�Ϊ����ˮ�ܽ⣬���������CaCl2��Һʹ������ȫ�����ú����ϲ���Һ�еμӷ�̪��Һ������Һ��죬���������NaOH������Һ����ɫ���������û��NaOH
�������ڽ������ʱ�����ȷ������п�������⣬Ȼ����ѧ����֪ʶ������������֪ʶ���з������
��2����������������Һ�Լ��ԣ���̼����������κ��������ԾͿ��Լ���
����⣺̼���������Ⱥ��ֽ��̼���ơ�ˮ�Ͷ�����̼���������������Ⱥ�ֽ⣬���Ⱥ��Թ��ڱ�����ɫҺ�Σ�������ɫ���壬ʯ��ˮ����ǣ�����һ������̼�����ƣ�
�������ƺ����ᷴӦ�����������壬̼���ƺ����ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼��
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ NaHCO3��Ӧ�Ļ�ѧ����ʽ��2NaHCO3  Na2CO3+H2O+CO2�� Na2CO3+H2O+CO2�� |
| ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� | ��������� | ʵ�����ʣ���ɫ������һ������ Na2CO3����Ӧ�Ļ�ѧ����ʽNa2CO3+2HCl=2NaCl+H2O+CO2�� |
�ʴ�Ϊ����ˮ�ܽ⣬���������CaCl2��Һʹ������ȫ�����ú����ϲ���Һ�еμӷ�̪��Һ������Һ��죬���������NaOH������Һ����ɫ���������û��NaOH
�������ڽ������ʱ�����ȷ������п�������⣬Ȼ����ѧ����֪ʶ������������֪ʶ���з������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

