��Ŀ����
������ѧϰ����Ҫ������С���ڸ�ϰ���������ʱ�����ɳ������������ѧ���ʡ�
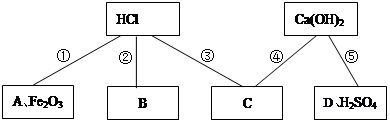
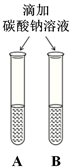
��1��Ϊ����֤���ʢ٣�С�콫��ɫʯ����Һ�μӵ�������Һ�У���Һ�� ɫ��
��2��ͼ��A����ʾ����������� ������д��һ������ʽ ��
��3����������ʢ۾��������������������⣬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4���������ʢݣ�������ʵ������ȡ������̼��д������ʽ ��
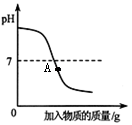
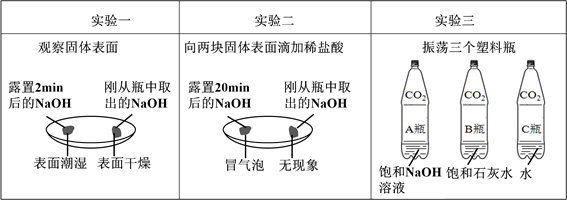
��5��þ��п����������֤��������ʢڣ�С��Ҫ̽��þ��п�����ᷴӦ�Ŀ�������Ҫ���Ʋ��䣨��ͬ������ ������ţ���

��1��Ϊ����֤���ʢ٣�С�콫��ɫʯ����Һ�μӵ�������Һ�У���Һ�� ɫ��
��2��ͼ��A����ʾ����������� ������д��һ������ʽ ��
��3����������ʢ۾��������������������⣬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4���������ʢݣ�������ʵ������ȡ������̼��д������ʽ ��
��5��þ��п����������֤��������ʢڣ�С��Ҫ̽��þ��п�����ᷴӦ�Ŀ�������Ҫ���Ʋ��䣨��ͬ������ ������ţ���
| A�����ֽ�������״ | B��������������� |
| C����Ӧ�����Ĵ�С | D���¶� |
��1���죻��2���NaOH+HCl=NaCl+H2O����3��Fe2O3+6HCl=2FeCl3+3H2O����4��CaCO3+2HCl=CaCl2+H2O+CO2�� ��5��ABD
�����������1��Ϊ����֤���ʢ٣�С�콫��ɫʯ����Һ�μӵ�������Һ�У���Һ���ɫ������������ʵ����ݿ�֪��2��ͼ��A����ʾ����������Ǽ�����ᷴӦ�ķ���ʽΪΪ��NaOH+HCl==NaCl+H2O����3����������ʢ۾��������������������⣬��ѧ����ʽΪ��Fe2O3+6HCl=2FeCl3+3H2O����4���������ʢݣ�������ʵ������ȡ������̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2������5��þ��п����������֤��������ʢڣ�С��Ҫ̽��þ��п�����ᷴӦ�Ŀ�������Ҫ���Ʋ��䣨��ͬ������Ϊ��������״������������������¶ȵ�.

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
�����Ŀ
 2NaOH + H2�� + Cl2����
2NaOH + H2�� + Cl2����