��Ŀ����
����Ŀ��ijѧ��д������6����ѧ����ʽ��
A Mg��O2=MgO2
B 2P2��2O5![]() 2P2O5
2P2O5
C Cu2(OH)2CO3=CuO����H2O��CO2��
D 3Fe��2O2��=Fe3O4
E Zn��H2SO4=ZnSO4��H2
F CuO��H2=Cu����H2O
����(�������)��
(1)��ѧʽд������______��
(2)��ѧʽ��ȷ�������������غ㶨�ɵ���______��
(3)��Ӧ����Ӧ��ע����δע������______��
(4)��������������ʹ�ò�������©����______��
���𰸡�AB C ACDF CDEF
��������
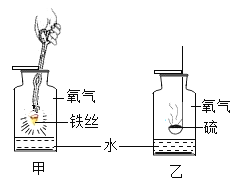
A þ����������ȼ�����ɵ�����þ�Ļ�ѧʽΪMgO������MgO2�����ڻ�ѧʽд�����Ҹ÷�Ӧ�������ǵ�ȼ����ѧ����ʽ��û�б����
B ���Ļ�ѧʽΪP������P2�����ڻ�ѧʽд����
C ��ʽ̼��ͭ�ڼ��ȵ������·ֽ���������ͭ��ˮ�Ͷ�����̼���û�ѧ����ʽ��ûע����Ӧ�������ҷ�Ӧǰ��Cu��Oԭ�ӵ���Ŀǰ��ͬ�������������غ㶨�ɣ��������ɵ�����ͭ�ǹ��壬�뷴Ӧ���״̬��ͬ������ꡰ������
D ���������ڵ�ȼ�������·�Ӧ�����������������û�ѧ����ʽ��û��ע����Ӧ��������������š�����Ӧ����������������Ļ�ѧʽ�ĺ��棬���ܱ��ڷ�Ӧ�����������ʵĻ�ѧʽ���棬����������������ʹ�ò�����
E п�����ᷴӦ��������п�������������������壬���������ʵ�״̬��ͬ��Ӧ���仯ѧʽ������������������ѧ����ʽ��û�б�������ڡ���������������©��
F ����ͭ�������ڼ��ȵ������·�Ӧ����ͭ��ˮ�����û�ѧ����ʽ��û�б����Ӧ�������ȣ������ɵ�ͭ�ǹ��壬�뷴Ӧ������ͭ��״̬��ͬ��������������������������ʹ�ò����������Ϸ�����֪��
��1����ѧʽд������AB������AB��
��2����ѧʽ��ȷ�������������غ㶨�ɵ���C������C��
��3����Ӧ����Ӧ��ע����δע������ACDF������ACDF��
��4����������������ʹ�ò�������©����CDEF������CDEF��

����Ŀ����Ƕȵ���ʶ��Һ�����������Ǹ��õ��˽��������������е���Ҫ���á�
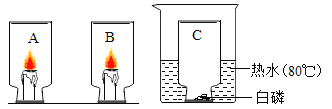
��1������ʯ����������ˮ��ϣ���ֽ�����á��ϲ���ҺΪ����ʯ��ˮ������_______________���²�δ��ȫ��Һ�IJ���δʯ���飬����_______________��
A��Һ B����Һ C����Һ
��2����������˵��![]() ����Һ�Ǿ�һ���ȶ�����____________��
����Һ�Ǿ�һ���ȶ�����____________��
A��Һ��������ɫ��ͬ B��Һ��������������������ͬ
C��Һ���ú���ɫ���� D����![]() ��Һ����ɫ��������
��Һ����ɫ��������
��3����һ�������������ʷֱ�Ͷ�뵽100gˮ�г���ܽ⣬����������Һ����������������
�������� | Ͷ������ |
| ������������ |
�Ȼ��� |
|
| _____ |
����� |
|
| _____ |
���� |
|
| _____ |
��4��20��ʱ��ij�����ڲ�ͬ������ˮ���ܽ�ﵽ����ʱ�����ʵ�������ˮ��������ϵ��ͼ��ʾ��
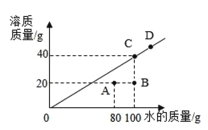
��![]() ʱ�������ʵ��ܽ����__________________���ʡ�
ʱ�������ʵ��ܽ����__________________���ʡ�
����D���Ӧ����120gˮ������������Ϊ____________g
����ͼ��֪��ҺA��ת���B��״̬����ͨ�������ܼ�����20gˮ���ķ�ʽʵ�֡�����Һ��B��ת���C��״̬����ͨ��_____________��ʽʵ�֣�