��Ŀ����
ij��ȤС���ľ̿��ԭ����ͭ���ɵ�����ɷֽ���ʵ��̽����
��������⣩̼������ͭ��Ӧ��������ʲô���壿
����������裩
����٣�ֻ����CO2������ڣ�_____������ۣ�������CO������CO2
���������ϣ�
�ٽ�����������Һ���Ȼ�����ֽ��CO2�ޱ仯��������COҲ������������
�ڵ����������ж���
��ʵ����֤��
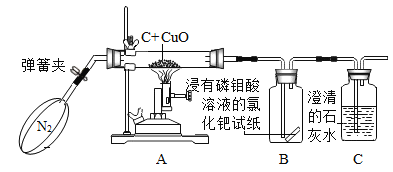
С��ͬѧ�������ͼ��ʾ��ʵ��װ�ã�
����һ�����ɼУ���ͨһ�����������ĵ�����
��������رյ��ɼУ���ȼ�ƾ��ƶԲ����ܼ��ȣ�
��������ʵ�������ϴװ�ú�������
��1��ʵ�鿪ʼʱ���Ƚ�����ͨ��ʵ��װ��һ��ʱ���ټ��ȵ�Ŀ����_____��
��2����Aװ�õIJ����ܼ��м���һ��ʱ�䣬�������к�ɫ��ĩ��ɺ�ɫ��Bƿ����ֽ������C�г���ʯ��ˮ����ǡ�д��C�з�����Ӧ�Ļ�ѧ����ʽ_____��
��ʵ����ۣ�
��3��ͨ����ʵ������ķ�������������_____������ţ�����ȷ�ġ�
����˼�뽻����
ʵ�������ͬѧ���ֲ������ڱڸ��ŵ�ͭ��ˮ������ϴ�ɾ���ͨ���������ϻ�����������ͭ�ķ�����
����Ũ���Ტ�ȣ�Cu+2H2SO4 ��Ũ��
����Ũ������ͭ��Ӧ��Cu+4HNO3��Ũ����Cu��NO3��2+2NO2+2H2O
�����Ȼ�����Һ��Cu+2FeCl3��2FeCl2+CuC12
��4���������ͭ����ѷ�����_____������ţ���������_____��
����չ��������һ���Ѳ��ֱ��ʵ�Ca��OH��2��������CaCO3��������Ʒ���Ѹ���ƷͶ������ˮ�У���ֽ����ͨ�������̼�����������뷴Ӧ�Ķ�����̼������ϵ��ͼ��ʾ��
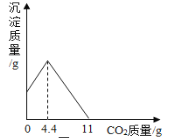
����ʾ��CO2+CaCO3+H2O��Ca��HCO3��2��
������Ʒ��̼��Ƶ�����������______��д��������̣������ȷ��0.1%��
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�����ʵ������ܴﵽʵ��Ŀ�ĵ��ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ��ȥCO2��������CO | ��ȼ |
B | ��ȥCuSO4��Һ�е�����FeSO4 | ����������ͭ�� |
C | �������ʯ��ˮ��NaOH��Һ | ����ϡ���� |
D | ����KNO3��NH4Cl | ȡ������ʯ�һ����ĥ������ζ |
A. A B. B C. C D. D


 �μ�Һ�� B.
�μ�Һ�� B.  ���Թ����Һ����� C.
���Թ����Һ����� C.  ȡ��ҩƷ D.
ȡ��ҩƷ D.  ��ȼ�ƾ���
��ȼ�ƾ���