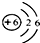��Ŀ����
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݡ�
(1)����̼��������������������ģ���Ҫ��Ϊ�˼���________(�ѧʽ)���ŷ�����
 (2)��ͼΪԪ�����ڱ��е�һ������˵������ȷ����
(2)��ͼΪԪ�����ڱ��е�һ������˵������ȷ����
______(����)��
A��̼Ԫ�����ڷǽ���Ԫ��
B��̼ԭ�Ӻ���������Ϊ6
 C.̼Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
C.̼Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
D��̼�����ԭ������Ϊ12.01
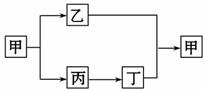 (3)�ס��ҡ��������dz��л�ѧ�����Ļ���������к���̼Ԫ�أ������������������֮������ͼ��ʾ��ת����ϵ(�������ʺͷ�Ӧ��������ȥ)����Ļ�ѧʽΪ______________________________________��
(3)�ס��ҡ��������dz��л�ѧ�����Ļ���������к���̼Ԫ�أ������������������֮������ͼ��ʾ��ת����ϵ(�������ʺͷ�Ӧ��������ȥ)����Ļ�ѧʽΪ______________________________________��
��ת��Ϊ���Ļ�ѧ����ʽΪ___________________________________________��
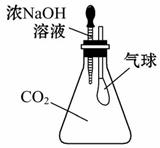
(4)��ͼ��ijȤζʵ��װ��ͼ����ѹ��ͷ�ιܺɹ۲쵽�����ʹ�������������ԭ��д����ѧ����ʽ:______________________________________��
___________________________________________________________________��
(5)������ͼװ�ÿ���CO��ԭFe2O3��ʵ�飬������÷�Ӧ���ɵ��� ������֪��Aװ����ȡ��CO�����л���������CO2��
������֪��Aװ����ȡ��CO�����л���������CO2��
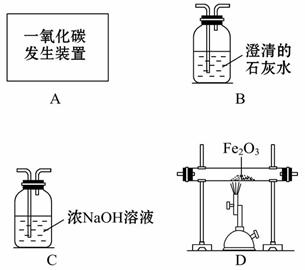
��CO��Fe2O3��Ӧ�Ļ�ѧ����ʽΪ______________________��
������ͨ��װ�õ� ˳����A��__________(װ�ò����ظ�ʹ��)��
˳����A��__________(װ�ò����ظ�ʹ��)��
�۴ӻ����Ƕȿ��ǣ�������װ�õĸĽ���ʩ��________ ___________________��
___________________��
��������(1)����̼����ָ�ϵ�(����)����������(������̼Ϊ��)�ŷţ�
(2)��ͼʾ��Ϣ֪��̼Ԫ�ص�������Ϊ6�������ԭ�Ӻ���ĵ�����ҲΪ6��C���еĺ����������8���ʲ���ȷ��(3)��ס��Ҷ�����̼Ԫ�أ���������������Ҽ��ֽܷ�õ��Һͱ����ʼ�Ϊ̼��ƣ����Ƕ�����̼�����������ƣ���ת��Ϊ������������ˮ��Ӧת��Ϊ�������ƣ�(4)�������̼��������������Һ��Ӧ��������ƿ�����������٣��ڲ���ѹ��С��������ѹ�Ὣ����ѹ�����Ӷ�ʹ�������ͣ�(5)������֪����ʵ���Ҫ��CO��ԭ����������Ҫ���鷴Ӧ�IJ������Ҫ������������Ƕ�����̼������A�õ���CO���Ѻ��ж�����̼����ˣ��ڻ�ԭ������֮ǰ���Ƚ������еĶ�����̼��ȥ��Ȼ������ɻ�ԭʵ�飬����ټ��������̼�����ɣ�ʵ���У���������������Һ��ȥ������̼���ó����ʯ��ˮ���������̼�Ĵ��ڣ���ʵ��װ�õ�����˳��Ϊ��A��C��D��B����ͼʾ����D��ȱ��β������װ�á�
�𰸣�(1)CO2 (2)C (3)CaCO3
CaO+H2O====Ca(OH)2
(4)CO2��ŨNaOH��Һ���գ���ƿ�ڵ���ѹ��С��������ѹ������ƿ�ڵ���ѹ��ʹ�����ʹ�
2NaOH+CO2====Na2CO3+H2O
(5)��Fe2O3+3CO 2Fe+3CO2 ��C��D��B
2Fe+3CO2 ��C��D��B
�۽�β����ȼ������ռ���

 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�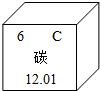 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�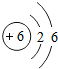
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�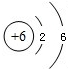 D��̼�����ԭ������Ϊ12.01
D��̼�����ԭ������Ϊ12.01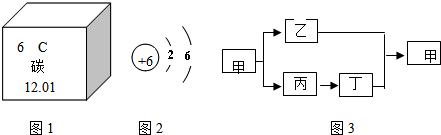
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�