��Ŀ����
����Ŀ��ѧУʵ���Ҹոչ�����һ������ʯ����ѧ��ȤС��ͨ��ʵ��ⶨ���ִ���ʯ��̼��Ƶĺ�������������ϡ������뵽20g����ʯ�У��Ѳ�����CO2������������NaOH��Һ���գ�ͬʱ����2������NaOH��Һ���ӵ�������������±���ʾ��
ʱ��/s | 0 | 20 | 40 | 60 | 80 | 100 | 120 |
���ӵ�����/g | 0 | 3.0 | 5.0 | 6.0 | 6.6 | 6.6 | 6.6 |
�Իش��������⣺
��1����������ֽ�ϣ��Է�Ӧʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�����ܹ��������������������ʱ��仯���ɵĹ�ϵ���ߣ� 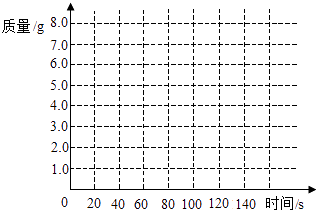
��2���ӱ��п��Կ�����20g����ʯ��Ʒ�����ᷴӦ��������ɵ�CO2g��д��NaOH��Һ����CO2����Ļ�ѧ����ʽ ��
��3���������ִ���ʯ�������ɷֲ������ᷴӦ���������ʯ��Ʒ��̼��Ƶ�����������
���𰸡�
��1���⣺����ͼ��ʾ��

��2���⣺6.6��2NaOH+CO2�TNa2CO3+H2O
��3���⣺�����ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+ | CO2�� |
100 | 44 |
x | 6.6g |
![]() =
= ![]() ��
��
x=15g��
����ʯ��Ʒ��̼��Ƶ���������Ϊ�� ![]() ��100%=75%��
��100%=75%��
�𣺴���ʯ��Ʒ��̼��Ƶ���������Ϊ75%��
���������⣺��1���Է�Ӧʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�ܹ��������������������ʱ��仯���ɵĹ�ϵ��������ͼ��ʾ��  ;��2���ӱ��п��Կ�����20g����ʯ��Ʒ�����ᷴӦ��������ɵ�6.6gCO2��
;��2���ӱ��п��Կ�����20g����ʯ��Ʒ�����ᷴӦ��������ɵ�6.6gCO2��
NaOH��Һ����CO2����ʱ����Ӧ����̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2�TNa2CO3+H2O��
���6.6��2NaOH+CO2�TNa2CO3+H2O��
�����㾫����������Ŀ����֪���������ø��ݻ�ѧ��Ӧ����ʽ�ļ�������֪ʶ���Եõ�����Ĵ𰸣���Ҫ���ո����ʼ�������=ϵ������Է�������֮�ȣ�

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ����ѧ�������������ᷢչ�����˾��ס����ж�ijһ����֪ʶ���ɶ���ȷ��һ����
A�����ʶ | B�����ʼ��� |
��������һһ�������� ����ȱпԪ��һһ��״���״� | ͭƬ����Ƭһһ�۲���ɫ ������һһ �ô����� |
C����ȫ��ʶ | D���������� |
�������Ż�һһ���Ը��Ϲ������ �����̺ܴ�ʱһһ��ʪë����ס�ڱ� | ���ٰ�ɫ��Ⱦһһ��ʹ��������Ʒ ����������Ⱦһһ��ʹ�û�ʯȼ�� |
A. A B. B C. C D. D