��Ŀ����
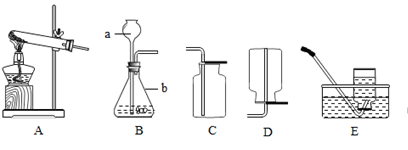
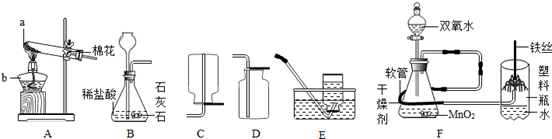
ij�о���ѧϰС������������װ�ý������������ȡ��̽���������������ա�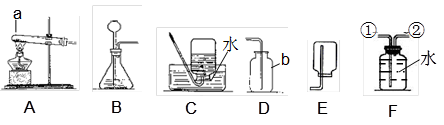
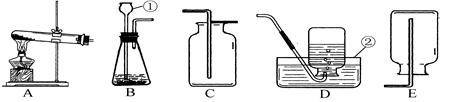
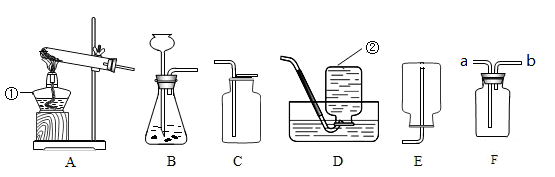
��1��д��ͼ������b������ b ��
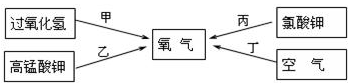
��2��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��Ӧѡ������巢��װ���� ��
��3��ʵ���Ҳ��ü�������غͶ������̵Ĺ���������ȡ��������Ӧ�Ļ�ѧ����ʽ�� ������ʢ��ˮ��Fװ���ռ�������Ӧ�ӵ��� ����ٻ�ڣ�ͨ�롣
��4��������һ����ɫ����ζ��������ˮ�����壬ʵ�����ü�����ˮ�����ƺͼ�ʯ�ҵĹ�������ķ�����ȡ���飬��ʵ������ȡ���ռ�����Ӧѡ���װ������� ��
�� ����ƿ �� Zn+H2SO4��Zn SO4+H2�� B
��2KClO3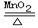 2KCl+ 3O2�� �� �� AC��AE ��AF���㣺
2KCl+ 3O2�� �� �� AC��AE ��AF���㣺
���������������1����Ϥ�����������˽����ƣ�ͼ��b�������ռ�����ļ���ƿ��
��2��ʵ������ȡ�����Ļ�ѧ����ʽ�ǣ�Zn+H2SO4=Zn SO4+H2�����˷�Ӧ������п��ϡ���᳣����ȡ���������ڹ�Һ�����ȷ�Ӧ������װ��B��Ϊ����װ�ã�
��3��ʵ���Ҽ�������غͶ���������ȡ�����Ļ�ѧ����ʽ�ǣ�2KClO3=2KCl+3O2������ʢ��ˮ��Fװ���ռ��������൱����ˮ���ռ�����������ɴӢڽ��룬ˮ���Ӣ��ų���
��4������ȡ�����Ǽ�����ˮ�����ƺͼ�ʯ�ҵĹ�������ǹ̹̼��ȷ�Ӧ����ѡ�õķ���װ����A���������ܶ�С�ڿ������ܶȣ�����������ˮ������C��ˮ���ռ�����E�����ſ������ռ���
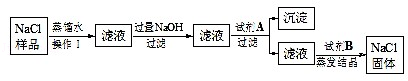
���㣺 ���������ʵ�����Ʒ������顢�����뾻��