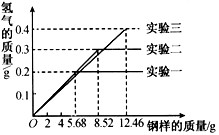
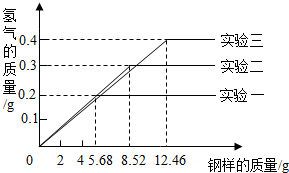
��Ŀ����
ij��ѧС���ͬѧ���������������飮���dz�ȡ�����ͬ��������ͬ�����ݸֵ���Ʒ������ֵ���Ʒ��ֻ������̼�����ֱ����100g 19.6%���������ʵ�飨���±�������ַ�Ӧ���õõ���ʵ�����ݻ��Ƴ���ͼ��ʾ��ͼ��| ʵ����� | ����������/g | 19.6%���������/g |
| һ | 5.68 | 100g |
| �� | 8.52 | 100g |
| �� | 12.46 | 100g |
�Իش�
��1��ʵ��һ�вμӷ�Ӧ����������Ϊ���ٿˣ�
��2��ϡ������ȫ��Ӧ��ʵ���У���Ӧ��������Һ�����ʵ����������Ƕ��٣�
��������1���Ա�ʵ��һ��ʵ����ɵã�ʵ��һ��5.68g������ϡ������ȫ��Ӧ�������������ᷴӦ�������������������Ļ�ѧ����ʽ���ɷ�Ӧ�ų������������ɼ���μӷ�Ӧ����������
��2����ʵ��һ�����ݿ�֪��5.68g������ȫ��Ӧ�ų�����0.2g��ͬ����ɵĸ���11.36g��ȫ��Ӧ�ɷų�����0.4g����ʵ����ȡ����12.46gʱ����õ�����ֻ��0.4g��˵������δ��ȫ��Ӧ����ϡ����㣬ϡ������ȫ��Ӧ����ʱ������ҺΪ����������Һ�����ʵ���������=
��100%��������������Ҫ���ݷ�Ӧ�Ļ�ѧ����ʽ�������������������㣻����Ӧ����Һ�����ɸ��������غ���м��㣮
��2����ʵ��һ�����ݿ�֪��5.68g������ȫ��Ӧ�ų�����0.2g��ͬ����ɵĸ���11.36g��ȫ��Ӧ�ɷų�����0.4g����ʵ����ȡ����12.46gʱ����õ�����ֻ��0.4g��˵������δ��ȫ��Ӧ����ϡ����㣬ϡ������ȫ��Ӧ����ʱ������ҺΪ����������Һ�����ʵ���������=
| �������������� |
| ��Ӧ����Һ���� |
����⣺��1����ʵ��һ�вμӷ�Ӧ����������Ϊx
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.2g
=
x=5.6g
��2��ʵ������100gϡ������ȫ��Ӧ���跴Ӧ����FeSO4������Ϊy��������������Ϊz
Fe+H2SO4�TFeSO4+H2��
56 152 2
z y 0.4g
=
y=30.4g
=
z=11.2g
������Һ�����ʵ���������=
��100%=27.4%
�𣺣�1��ʵ��һ�вμӷ�Ӧ����������Ϊ5.6g��
��2��ϡ������ȫ��Ӧ��ʵ�����У���Ӧ��������Һ�����ʵ�����������27.4%��
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.2g
| 56 |
| x |
| 2 |
| 0.2g |
��2��ʵ������100gϡ������ȫ��Ӧ���跴Ӧ����FeSO4������Ϊy��������������Ϊz
Fe+H2SO4�TFeSO4+H2��
56 152 2
z y 0.4g
| 152 |
| y |
| 2 |
| 0.4g |
| 56 |
| z |
| 2 |
| 0.4g |
������Һ�����ʵ���������=
| 30.4g |
| 100g+11.2-0.4g |
�𣺣�1��ʵ��һ�вμӷ�Ӧ����������Ϊ5.6g��
��2��ϡ������ȫ��Ӧ��ʵ�����У���Ӧ��������Һ�����ʵ�����������27.4%��
���������������غ㶨�ɣ�ϡ������ȫ��Ӧ��ʵ����������Һ����=ϡ��������+�����вμӷ�Ӧ��������-����������������

��ϰ��ϵ�д�
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д�
�����Ŀ
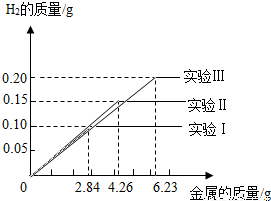
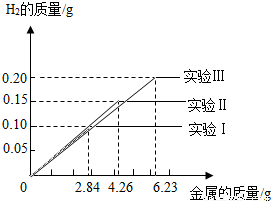
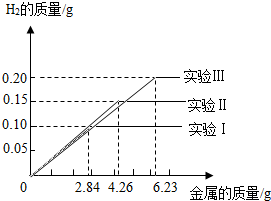 ij��ѧС���ͬѧ���������������飮���dz�ȡ�����ȫ��ͬ������Ϊ2.84g��4.26g��6.23g���������Ͻ���Ʒ����������ֻ������̼������������Ʒ�зֱ����94.6gϡ�����н���ʵ�飨ʵ����I������ַ�Ӧ���õ���ʵ�����ݻ��Ƴ���ͼ���Իش�
ij��ѧС���ͬѧ���������������飮���dz�ȡ�����ȫ��ͬ������Ϊ2.84g��4.26g��6.23g���������Ͻ���Ʒ����������ֻ������̼������������Ʒ�зֱ����94.6gϡ�����н���ʵ�飨ʵ����I������ַ�Ӧ���õ���ʵ�����ݻ��Ƴ���ͼ���Իش�