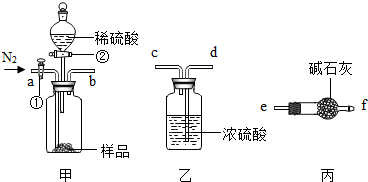
��Ŀ����
��2008?�人��Ԫ�����ڱ��У��˵������1��18��Ԫ�ص�ԭ�ӽṹʾ��ͼ��ͼ��
��1����ͼ���Կ�����ϡ������Ԫ�ء�����Ԫ�غͷǽ���Ԫ�ص�ԭ�������ĵ�����Ŀ�����й��ɣ����У�����Ԫ��ԭ�ӵ��������ӵ���Ŀһ��
��2����Ԫ��ԭ�ӵ���������
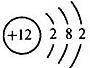
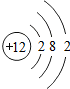
��3��ijԪ�ص�ԭ�ӽṹʾ��ͼΪ �����Ԫ��ԭ�ӵĺ˵����Ϊ
�����Ԫ��ԭ�ӵĺ˵����Ϊ
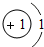 �� |
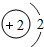 �� | ||||||
 � |
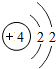 �� |
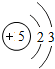 �� |
 ̼ |
 �� |
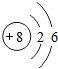 �� |
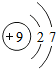 �� |
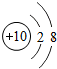 �� |
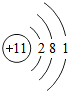 �� |
 þ |
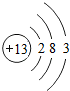 �� |
 �� |
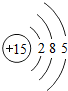 �� |
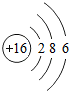 �� |
 �� |
 � |
��
��
4����ѡ����ڡ�����С�ڡ��������ڡ�������2����Ԫ��ԭ�ӵ���������
2
2
����ͨ������¸�Ԫ�صĻ�ѧ�����ȶ�
�ȶ�
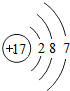
��ѡ��ȶ����������ȶ���������3��ijԪ�ص�ԭ�ӽṹʾ��ͼΪ
 �����Ԫ��ԭ�ӵĺ˵����Ϊ
�����Ԫ��ԭ�ӵĺ˵����Ϊ37
37
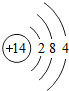
���������Ԫ�ص�ԭ�ӽṹʾ��ͼ�жϣ�1-18��Ԫ�������Ԫ�ػ�ѧ���������Ƶ�һ��Ԫ������
��
�������ơ�Ԫ�ط��ž��ɣ�����������1������ͼʾ��������Ԫ��ԭ�ӵ�����������Ŀ�Ĺ��ɣ�
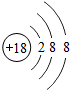
��2�����ݺ�Ԫ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ������
��3�����ݺ˵�������ں������������������Ԫ��ԭ��������������ͬ��ѧ�������ƽ��з�����
��2�����ݺ�Ԫ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ������
��3�����ݺ˵�������ں������������������Ԫ��ԭ��������������ͬ��ѧ�������ƽ��з�����
����⣺��1����ͼ���Կ���������Ԫ��ԭ�ӵ��������ӵ���Ŀһ��С��4����
��2���Ӻ�Ԫ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ���Կ�������Ԫ��ԭ�ӵ���������2����ͨ������¸�Ԫ�صĻ�ѧ�����ȶ���
��3�����ݺ˵�������ں����������֪�����Ԫ��ԭ�ӵĺ˵����Ϊ2+8+18+8+1=37������Ԫ��ԭ��������������ͬ��ѧ�������ƣ�1-18��Ԫ�������Ԫ�ػ�ѧ���������Ƶ�һ��Ԫ�����ƣ�
�ʴ�Ϊ����1��С�ڣ���2��2���ȶ�����3��37���ƣ�
��2���Ӻ�Ԫ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ���Կ�������Ԫ��ԭ�ӵ���������2����ͨ������¸�Ԫ�صĻ�ѧ�����ȶ���
��3�����ݺ˵�������ں����������֪�����Ԫ��ԭ�ӵĺ˵����Ϊ2+8+18+8+1=37������Ԫ��ԭ��������������ͬ��ѧ�������ƣ�1-18��Ԫ�������Ԫ�ػ�ѧ���������Ƶ�һ��Ԫ�����ƣ�
�ʴ�Ϊ����1��С�ڣ���2��2���ȶ�����3��37���ƣ�
�����������ؼ���Ҫ֪���˵���������������������Ĺ�ϵ������ȶ��ṹ���ص㣬�˽�Ԫ��ԭ��������������ͬ��ѧ�������ƣ�

��ϰ��ϵ�д�
�����Ŀ