��Ŀ����
����Ŀ����ѧ���Ѿ���ʵ���������������������(ice ��)�����ֱ���Ҫ�ں��д���ˮԴ�ͼ��ߵ�ѹǿ�²��ܹ����ɣ�ˮ���ӻ��������л��ų�һ�зdz������й���ı���������һ����������������������˵������ȷ����
A. ���ߺ�ˮ�Ļ�ѧ���ʲ���ȫ��ͬ
B. �����з���֮��ľ������ͨ���е�С
C. ��ˮ��һ�������¿����γɱ���
D. �����еķ�������ͣϢ���˶�
���𰸡�A
��������A��ͬ�ַ���������ͬ����ͬ�������ʲ�ͬ����7��ˮ������ˮ���ӹ��ɣ����Ի�ѧ������ͬ����A˵������ B����Ҫ�ں��д���ˮԴ�ͼ��ߵ�ѹǿ�²��ܹ����ɣ�����ѹǿ����7�з���֮��ľ����С������ͨ���е�С����B˵����ȷ�� C����7��Ҫ�ں��д���ˮԴ�ͼ��ߵ�ѹǿ���γɣ���ˮ�к��д�����ˮ����C˵����ȷ��D�������ڲ����˶������Ա�7�еķ�������ͣϢ���˶�����D˵����ȷ����ѡA��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ҹ���ѧ�Һ�°����ĺ����ƼΪ���ҵ�����ķ�չ�����˽ܳ��Ĺ��ס����Ʊ�����Ĺ������漰Na2CO3��NaCl��NH4Cl��NaHCO3�����ʡ����ϱ������ݻش��������⣺
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | Na2CO3 | 7 | 12.2 | 21.8 | 39.7 | 48.8 | 47.3 | 46.4 |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | |
NaHCO3 | 6.9 | 8.2 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | |
(1)�������������У��ܽ������ڿ��ܵ�������__________��
(2)��50��ʱ����100gˮ�м���48gNa2CO3��ֽ���������Һ����Ϊ___________ �������ձ��и������������䣬������40��ʱ��������Һ���ʵ�����������_________(������С���������������������)��
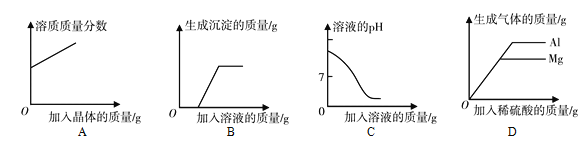
(3)����ݱ��������ݣ�����ͼ�л��Ƴ�̼���Ƶ��ܽ�����ߡ�
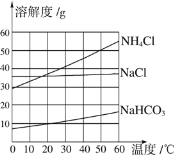 __________
__________
(4)�����ܽ�����ش��������⣺
��60��ʱ����������������������������зֱ��ˮ��ɱ�����Һ��������Һ�����ɴ�С��˳����_____________________________��
�ں����Ƽ�Ĺؼ������ǣ�����ʳ��ˮ��ͨ�백�����Ƴɱ��Ͱ���ˮ������ˮ���ն�����̼������̼�����ƺ��Ȼ�泥��仯ѧ����ʽΪNaCl+NH3��H2O+CO2��NH4Cl��NaHCO3���������ɵ�̼�����ƺ��Ȼ�泥�����������������______________��������_________________��