��Ŀ����
��ȡ12.5gʯ��ʯ����Ҫ�ɷ�CaCO3����־������ˮ��Ҳ���μӷ�Ӧ�������ձ��У������м���50gϡ���ᣬ����ǡ����ȫ��Ӧ����Ӧ����������ձ���ʣ�����ʵ�������Ϊ58.1g���������ձ�����������������ܽ���Բ��ƣ���
�Լ��㣺
��1����ȫ��Ӧ�����ɶ�����̼�������Ƕ��ٿˣ�
��2��12.5g��Ʒ�к�CaCO3�����������Ƕ��٣�
��3����Ӧ��������Һ�����ʵ������Ƕ��٣��������ȷ��1%��
�Լ��㣺
��1����ȫ��Ӧ�����ɶ�����̼�������Ƕ��ٿˣ�
��2��12.5g��Ʒ�к�CaCO3�����������Ƕ��٣�
��3����Ӧ��������Һ�����ʵ������Ƕ��٣��������ȷ��1%��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���
��������1�����������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ������̼��������
��2������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼�����������̼��Ƶ������������������������㹫ʽ�������ʯ��ʯ��̼��Ƶ�����������
��3������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼������������Ȼ��Ƶ���������Ӧ��������Һ������Ϊ̼��Ƶ����������������֮�ͼ�ȥ������̼���������������������������㹫ʽ������������
��2������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼�����������̼��Ƶ������������������������㹫ʽ�������ʯ��ʯ��̼��Ƶ�����������
��3������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼������������Ȼ��Ƶ���������Ӧ��������Һ������Ϊ̼��Ƶ����������������֮�ͼ�ȥ������̼���������������������������㹫ʽ������������
����⣺��1������CO2������Ϊ��12.5g+50 g-58.1g=4.4g
�ʴ�Ϊ��4.4
��ʯ��ʯ��CaCO3������Ϊx�������Ȼ��Ƶ�����Ϊy
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 111 44
x y 4.4g
=
=
x=10g��y=11.1g
ʯ��ʯ��̼��Ƶ���������Ϊ��
��100%=80%
��������Һ������Ϊ��10g+50g-4.4g=55.6g
����������Һ��������������Ϊ��
��100%=20%
�𣺣�1��ʯ��ʯ��̼��Ƶ���������Ϊ80%��
��2������������Һ��������������Ϊ20%��
�ʴ�Ϊ��4.4
��ʯ��ʯ��CaCO3������Ϊx�������Ȼ��Ƶ�����Ϊy
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 111 44
x y 4.4g
| 100 |
| x |
| 111 |
| y |
| 44 |
| 4.4g |
x=10g��y=11.1g
ʯ��ʯ��̼��Ƶ���������Ϊ��
| 10g |
| 12.5g |
��������Һ������Ϊ��10g+50g-4.4g=55.6g
����������Һ��������������Ϊ��
| 11.1g |
| 55.6g |
�𣺣�1��ʯ��ʯ��̼��Ƶ���������Ϊ80%��
��2������������Һ��������������Ϊ20%��
���������ݻ�ѧ����ʽ���м���ʱ��ֻ��ʹ�ô�������������м��㣬�����ܰѻ���������ֱ�Ӵ��뻯ѧ����ʽ���м��㣬�йط�Ӧǰ����ٵ������ļ����dz��л�ѧ�����һ���ص����ݣ���Ӧǰ����װ�ü��ٵ�����һ��Ϊ���������������

��ϰ��ϵ�д�
 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
�����Ŀ
ijѧ��ȡһƿϡ���ᣬ�������װ�ڼס������ձ��У��ٳ�ȡ������ȵ�þ����п������þ��������ձ��У�п���������ձ��У�����Ӧ�������ּ��ձ���þ����ʣ�࣬���ձ���п����ȫ����Ӧ������ʵ�������ƶϣ����н�����ȷ���ǣ�������
| A�����ձ��в��������� |
| B�����ձ��в����������� |
| C���������ձ��в���������һ���� |
| D�����ձ��в������������������ձ��в���������-���� |
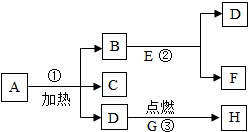
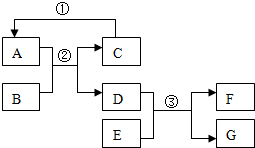 ��ͼA-G��Ϊ���� ��ѧ���������ʣ�����A��C�����Ԫ����ͬ�����壬B���������Ҫ�ɷ֣�GΪ��ɫ�������ʣ�������ͼ��ʾ��ת����ϵ��ͼ�еķ�Ӧ����������ȥ������ش�
��ͼA-G��Ϊ���� ��ѧ���������ʣ�����A��C�����Ԫ����ͬ�����壬B���������Ҫ�ɷ֣�GΪ��ɫ�������ʣ�������ͼ��ʾ��ת����ϵ��ͼ�еķ�Ӧ����������ȥ������ش�