��Ŀ����
��ͼ��ʾʵ�飬AΪһ����ɫ���̼�����ζ�����壬װ�â�Ӳ�ʲ�������װ��1.60�˺�ɫ��ĩB��A��B����������й�ʵ���������£�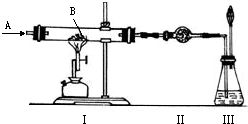
| ʵ������ | ���� | �ⶨ��� |
| ��ʹA������ͨ��ʵ��װ�â� | ���к�ɫ��ĩ��ȫ����� | ���в����ĺ�ɫ��ĩ������Ϊ1.12�� |
| �ڵ�A��B�ڼ����·�Ӧʱ | ���и���������ˮCuSO4�ޱ仯 | |
| ��ʵ�����ʱ������ȥ��Ϩ��ƾ��ƣ��������º���ֹͣͨA | ���г���ʯ��ˮ����� | ���г���1.00�� |
ԭ��������
��2��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ��
��3����Ӧ��װ�â��ڵ�Һ����������⣬���е�������
��4��װ�â��ɼ���ų������壬Ҫ�����ȼ��ԭ����
��������1���������֪��1.60�˺�ɫ��ĩB��Fe2O3������ɫ��ĩ������1.16�ˣ���������ϡ���ᷴӦ����һ����RSO4����R�����ԭ��������56��
��2����������ͭ�ޱ仯��˵����Ӧ��ˮ���ɣ���ԭ���õ���һ����̼�����������������Կ�����д��ѧ����ʽ��
��3���������֪��1.60�˺�ɫ��ĩB��Fe2O3������ɫ��ĩ������1.16�ˣ������֪�����ɵĶ�����̼������Ϊ1.12�ˣ�����������ij���ʯ��ˮ��Ӧ����������̼��ƣ�������ֻ����̼���1�ˣ����ԣ�������̼�ֺ�ˮ��̼�������̼����ƣ�
��4��β������һ����̼��������Ⱦ����������ȼ�գ�
��2����������ͭ�ޱ仯��˵����Ӧ��ˮ���ɣ���ԭ���õ���һ����̼�����������������Կ�����д��ѧ����ʽ��
��3���������֪��1.60�˺�ɫ��ĩB��Fe2O3������ɫ��ĩ������1.16�ˣ������֪�����ɵĶ�����̼������Ϊ1.12�ˣ�����������ij���ʯ��ˮ��Ӧ����������̼��ƣ�������ֻ����̼���1�ˣ����ԣ�������̼�ֺ�ˮ��̼�������̼����ƣ�
��4��β������һ����̼��������Ⱦ����������ȼ�գ�
����⣺��1���ɷ�����֪����ɫ��ĩBΪFe2O3����ɫ��ĩΪ�������R�����ԭ��������56��
��2���ɷ�����֪����ԭ���õ���һ����̼�����ԣ�Fe2O3+3CO
2Fe+3CO2�������֪�����ɵĶ�����̼������Ϊ1.12�ˣ�
��3���١�Ca��OH��2+CO2=CaCO3��+H2O�������֪�����Ķ�����̼0.88�ˣ�����̼���2�ˣ�
�ڡ�CaCO3+CO2+H2O=Ca��HCO3��2�������֪�����Ķ�����̼0.44�ˣ��õ�̼���1������̼����ƣ���������ֻ����1.00��̼��ƣ����װ�â��ڵ�Һ����������⣬���е������� ̼����ƣ�
��4��β������һ����̼��������Ⱦ����������ȼ�գ�����ֹ����Ⱦ������
�ʴ�Ϊ����1��56
��2��Fe2O3+3CO
2Fe+3CO2
��3��̼�����
��4����ȥβ���е�CO����ֹ����Ⱦ������
��2���ɷ�����֪����ԭ���õ���һ����̼�����ԣ�Fe2O3+3CO
| ||
��3���١�Ca��OH��2+CO2=CaCO3��+H2O�������֪�����Ķ�����̼0.88�ˣ�����̼���2�ˣ�
�ڡ�CaCO3+CO2+H2O=Ca��HCO3��2�������֪�����Ķ�����̼0.44�ˣ��õ�̼���1������̼����ƣ���������ֻ����1.00��̼��ƣ����װ�â��ڵ�Һ����������⣬���е������� ̼����ƣ�
��4��β������һ����̼��������Ⱦ����������ȼ�գ�����ֹ����Ⱦ������
�ʴ�Ϊ����1��56
��2��Fe2O3+3CO
| ||
��3��̼�����
��4����ȥβ���е�CO����ֹ����Ⱦ������
���������ո��ݻ�ѧ����ʽ����IJ���ͷ�������ȷ�ҳ����е�ͻ�ƿڣ�������ϣ�һ��Ҫ��֤�Ƿ���ȷ��

��ϰ��ϵ�д�
�����Ŀ
��ͼ��ʾʵ�飬AΪһ����ɫ���̼�����ζ�����壬װ�â�Ӳ�ʲ�������װ��1.60�˺�ɫ��ĩB��A��B����������й�ʵ���������£�
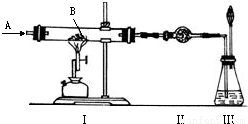
��1���������ú�ɫ��ĩ��������ϡ�����У�������H20.04�ˣ�ͬʱ����һ����RSO4����R�����
ԭ��������______��
��2��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ��______ 2Fe+3CO2

| ʵ������ | ���� | �ⶨ��� |
| ��ʹA������ͨ��ʵ��װ�â� | ���к�ɫ��ĩ��ȫ����� | ���в����ĺ�ɫ��ĩ������Ϊ1.12�� |
| �ڵ�A��B�ڼ����·�Ӧʱ | ���и���������ˮCuSO4�ޱ仯 | |
| ��ʵ�����ʱ������ȥ��Ϩ��ƾ��ƣ��������º���ֹͣͨA | ���г���ʯ��ˮ����� | ���г���1.00�� |
ԭ��������______��
��2��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ��______ 2Fe+3CO2
��ͼ��ʾʵ�飬AΪһ����ɫ���̼�����ζ�����壬װ�â�Ӳ�ʲ�������װ��1.60�˺�ɫ��ĩB��A��B����������й�ʵ���������£�
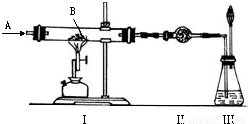
��1���������ú�ɫ��ĩ��������ϡ�����У�������H20.04�ˣ�ͬʱ����һ����RSO4����R�����
ԭ��������______��
��2��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ��______ 2Fe+3CO2

| ʵ������ | ���� | �ⶨ��� |
| ��ʹA������ͨ��ʵ��װ�â� | ���к�ɫ��ĩ��ȫ����� | ���в����ĺ�ɫ��ĩ������Ϊ1.12�� |
| �ڵ�A��B�ڼ����·�Ӧʱ | ���и���������ˮCuSO4�ޱ仯 | |
| ��ʵ�����ʱ������ȥ��Ϩ��ƾ��ƣ��������º���ֹͣͨA | ���г���ʯ��ˮ����� | ���г���1.00�� |
ԭ��������______��
��2��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ��______ 2Fe+3CO2


