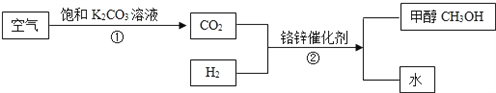
��Ŀ����
����Ŀ��ijʵ��С�鹺������ʳ�þ���ֲ������̼��ƣ���ǩ��ͼ1��Ϊ�ⶨ̼��Ƶ�����������ʵ��С�����������ʵ�飺
�ٳ�ȡһ������Ʒ����һ�ձ��У�
�����������ձ��м���50g�������ַ�Ӧ��
�ۻ��Ʒų��������ձ������������Ĺ�ϵͼ��ͼ2��������ܰ��ʾ��ͼ��58gΪ��Ӧ��ʼʱ�ձ������ʵ���������
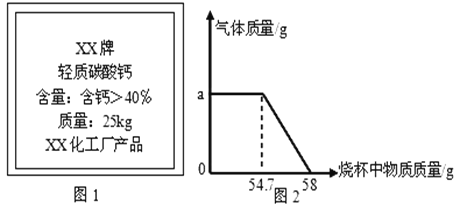
����㣬���ش������й����⣺
��1����Ӧ�����в���������������______g��
��2������Ӧ�����ĵ���������Ϊ50g������Ʒ��̼��Ƶ����������Ƕ��٣���д��������̣��������С�����2λ��
��3�����㻭�����Ĺ�����Ʒ�������������������仯��ϵͼ��
��4�������������̼�����Ʒ���������ʲ�����Ԫ��,����ͨ������ش��ǩ��ʾ�ĺ�����______������ȷ����ȷ����
���𰸡���1��3.3����2��93.75%����3�� ����4������ȷ
����4������ȷ
����������1�����������غ㶨�ɼ�ͼʾ�����н��
��2�������ձ��м���50g�����ᣬ58gΪ��Ӧ��ʼʱ�ձ������ʵ����������з���̼��Ƶ�������
��3���������Ĺ�����Ʒ�������������������仯��ϵ��ͼ��
��4���������������ıȽϣ����ɽ�á�
�⣺��1�����������غ㶨�ɼ�ͼʾ��֪����Ӧ�����в�������������Ϊ��58-54.7=3.3g���ʴ�Ϊ��3.3g��
��2������֪̼�����Ʒ��������8g��
����Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+CO2��+H2O
10044
x 3.3g![]()
���x=7.5g
��Ʒ��̼��Ƶ����������� ![]() ��100%=93.75%
��100%=93.75%
����Ʒ��̼��Ƶ�����������93.75%
��3���������Ĺ�����Ʒ�������������������仯��ϵ��ͼ��
��4����Ʒ�и�Ԫ�ص���������=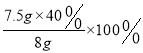 =37.5%��40%
=37.5%��40%
��Ϊ 37.5%��40%
ͨ�������֪��ǩ��ʾ�ĺ�����������ȷ��

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�����Ŀ�����г�ȥ���ʵķ����У���ȷ����
ѡ �� | ���ʣ�������Ϊ�������ʣ� | ��ȥ���ʵķ��� |
A | CO2��CO�� | ͨ��������ȼ |
B | H2O��H2O2�� | ����MnO2������ |
C | CaO��CaCO3�� | ��ˮ�ܽ⣬���� |
D | CuO��C�� | ����������ǿ�� |