��Ŀ����
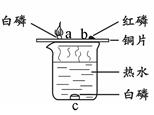
��7�֣���һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺

���ϣ�1.�ƾ��Ƽ����¶�Լ500��600 �棻
2. NaOH 318.4���ۻ����ֽ⣬1390 ����ڲ��ֽ⣻
3. Na2CO3+CaCl2��CaCO3��+ 2NaCl
4. Na2CO3��Һ�ʼ��ԣ�CaCl2��Һ������
��1�����ʵ�鱨������ݣ�
��2��Ϊȷ����ɫ��ĩ���Ƿ���NaOH�ķ����ǣ�ȡʵ�����ʣ���ɫ���������� ��

���ϣ�1.�ƾ��Ƽ����¶�Լ500��600 �棻
2. NaOH 318.4���ۻ����ֽ⣬1390 ����ڲ��ֽ⣻
3. Na2CO3+CaCl2��CaCO3��+ 2NaCl
4. Na2CO3��Һ�ʼ��ԣ�CaCl2��Һ������
��1�����ʵ�鱨������ݣ�
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ ��Ӧ�Ļ�ѧ����ʽ�� |
| ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� | | ʵ�����ʣ���ɫ������һ������ �� ��Ӧ�Ļ�ѧ����ʽ�� |
��1����NaHCO3 ��2NaHCO3��Na2CO3+ H2O+CO2 �����������
��Na2CO3��Na2CO3+2HCl��2NaCl+ H2O+CO2��
��2����ˮ�ܽ⣬���������CaCl2��Һʹ������ȫ�����ú����ϲ���Һ�еμӷ�̪��Һ������Һ��죬���������NaOH������Һ����ɫ���������û��NaOH��2�֣�
��Na2CO3��Na2CO3+2HCl��2NaCl+ H2O+CO2��
��2����ˮ�ܽ⣬���������CaCl2��Һʹ������ȫ�����ú����ϲ���Һ�еμӷ�̪��Һ������Һ��죬���������NaOH������Һ����ɫ���������û��NaOH��2�֣�
�����������1��
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ NaHCO3 �� ��Ӧ�Ļ�ѧ����ʽ��2NaHCO3=Na2CO3+ H2O+CO2 |
| ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� | ��������� | ʵ�����ʣ���ɫ������һ������ Na2CO3�� ��Ӧ�Ļ�ѧ����ʽ�ǣ�Na2CO3+2HCl=2NaCl+ H2O+CO2�� |
�������ƶ����ʣ�Ҫ�������ʵ����ʣ���ƺ�����ʵ�飬ͨ�����еķ�Ӧ������ȷ��������������Ϣ�������Ŀ�еĿ��㣬�ǽ����Ĺؼ���

��ϰ��ϵ�д�
 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�
�����Ŀ