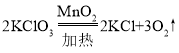��Ŀ����
����Ŀ���ᡢ����dz��л�ѧѧϰ����Ҫ���ݡ�
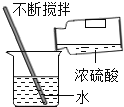
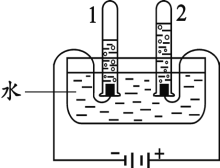
��1�����ᡢ����ȳ������ᶼ�������ƵĻ�ѧ���ʣ�����Ϊ������ˮ��Һ�ж������____________![]() �����ӷ���
�����ӷ���![]() ��
��
��2����84����Һ������Чɱ���¹ڲ�����ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ��

�ٸ�ƿ��84����Һ����NaClO������Ϊ_________g��
�ڹ�ҵ��������������������Һ��Ӧ����ȡNaClO��ͬʱ����ʳ�ε���Ҫ�ɷֺ�ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ_____________��
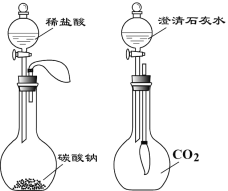
��3��ʵ������һƿ����¶���ڿ����е�����������Һ��Ϊȷ����ɷ֣�С��ͬѧ����������̽����
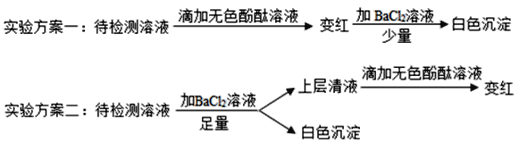
�ٸ���ʵ�鷽����ȷ����ƿ��Һ�����ʳɷ���ʲô_____________��
��ʵ�鷽��һ����ȷȷ�����ʳɷ֣���˵������_______________��
��ʵ����Ϊ��ֹNaOH��Һ���ʣ��ɲ�ȡʲô��ʩ_______________��
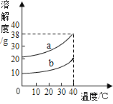
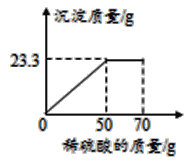
��4��ij���������п��ܺ���NaCl��![]() ��NaOH�е�һ�ֻ��֡�ijУ����С��ͬѧΪ��̽����ɷ֣�ȡ�ù�������30g��ȫ����ˮ�У������Һ��pHΪ7��Ȼ��70gijϡ����������Һ�У���ַ�Ӧ�����������������������Ĺ�ϵ��ͼ��ʾ�������ͼʾ�����֪ʶ�ش����⡣
��NaOH�е�һ�ֻ��֡�ijУ����С��ͬѧΪ��̽����ɷ֣�ȡ�ù�������30g��ȫ����ˮ�У������Һ��pHΪ7��Ȼ��70gijϡ����������Һ�У���ַ�Ӧ�����������������������Ĺ�ϵ��ͼ��ʾ�������ͼʾ�����֪ʶ�ش����⡣
�ٸù���������һ�������е�������__________![]() ��ѧʽ
��ѧʽ![]() ��
��
������ϡ���������ʵ�����������_________��
���𰸡�H+ 447 Cl2+2NaOH=NaClO+NaCl+H2O ̼���ƺ��������� �������ƺ�̼������Һ���Լ��ԣ�����ʹ��ɫ��̪��� �ܷⱣ�� NaOH 19.6%
��������
��1�����ǽ����������ȫ�������ӵĻ�������ᡢ����ȳ������ᶼ�������ƵĻ�ѧ���ʣ�����Ϊ������ˮ��Һ�ж�����������ӣ����H+
��2��NaClO������Ϊ1000mL��1.2g/mL��37.25%=447g���447������������������Һ��Ӧ����NaClOͬʱ����ʳ����Ҫ�ɷ��Ȼ��ƺ�ˮ��ѧ����ʽΪ��Cl2+2NaOH=NaClO+NaCl+H2O�����Cl2+2NaOH=NaClO+NaCl+H2O
��3���������Ʒ����ڿ��������Ͷ�����̼������Ӧ����̼���ƺ�ˮ�������ʣ�������������Һ��Ҫ�ܷⱣ�棬����������Һ���ʷ�Ϊ���������û�б��ʣ�����Ϊ�������ƣ����ֱ��ʣ�����Ϊ�������ƺ�̼���ƣ�ȫ�����ʣ�����Ϊ̼���ƣ��Ȼ������Ժ�̼���Ʋ�����ɫ������ʵ�鷽�������Ȼ���������ɫ����˵����̼���ƣ�ȡ�ϲ���Һ����ɫ��̪���˵����Һ�����������ƣ�ʵ�鷽��һ�������Ʊ��ʲ����ʼӷ�̪�����죬��Ϊ̼������Һ���Լ��Եģ�Ҳ��ʹ��ɫ��̪��죬�μ��Ȼ���������ɫ����˵����Һ�к���̼���ƣ������Ƿ�������������ȷ�������̼���ƺ��������ƣ��������ƺ�̼������Һ���Լ��ԣ�����ʹ��ɫ��̪��죻�ܷⱣ�棻
��4��pH����7˵����Һ�����Բ���NaOH,���NaOH
�⣺��ϡ����������������Ϊx����ͼ��֪������50gϡ����ʱǡ����ȫ��Ӧ

��ϡ����������������19.6%��

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
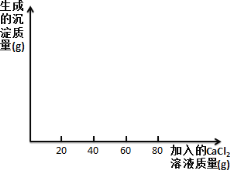
��ѧ����ϵ�д�����Ŀ��ijʳ�ü����Ҫ�ɷ��� Na2CO3�����к��������� NaCl��С��ͬѧΪ�ⶨ��ʳ�ü��� Na2CO3 ������������������������ʵ�飬ȡ 40 g ʳ�ü��ˮ��� 400g ����Һ������Һƽ����Ϊ�ķݣ�Ȼ��ֱ����һ������������ CaCl2 ��Һ��ʵ�����ݼ��±���
ʵ��һ | ʵ��� | ʵ���� | ʵ���� | |
ʳ�ü������/g | 10 | 10 | 10 | 10 |
����CaC12��Һ������/g | 20 | 40 | 60 | 80 |
���ɳ���������/g | 2.5 | 5 | m | 8 |
������������ݲ�����ش�
��1��m��________��
��2��40 g ��ʳ�ü���ɵ���Һ������ CaCl2 ��Һ��Ӧ��������ɳ�������Ϊ_____g��
��3����ʳ�ü��� Na2CO3 �����������Ƕ���_____����д��������̣��𰸾�ȷ�� 0.1%��
��4�������ʵ���ĵ����ݻ�ͼ�����Ϻ��ʵ���������_____��