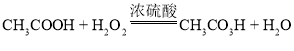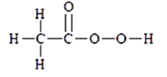��Ŀ����
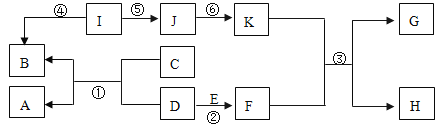
����Ŀ��A~K�dz��л�ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ��������ָ�������������AΪ��ɫ���壬B��C��Ԫ�������ͬ��I���������Ƽ���F��K�������ʳ���������ũҩ������Һ����ش��������⣺
��1������F�Ļ�ѧʽ��_____������J���׳�_____��
��2����Ӧ�ݵĻ�����Ӧ����Ϊ_____��
��3����Ӧ�۵Ļ�ѧ����ʽΪ_____��
��4��B��C���������ʵĻ����3.6g���������ȵ�D���ʳ�ַ�Ӧ�����ɵ�����ȫ��ͨ��80����������Ϊ20%������������Һ�г�����գ��������������Һ������Ϊ84.4�ˣ���ԭ�������C���ʵ�����Ϊ_____g��
���𰸡�CuSO4 ��ʯ�� �ֽⷴӦ ![]() 1.4
1.4
��������
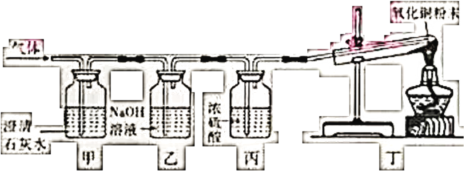
����Ŀ��ͻ�ƿڣ�F��K�������ʳ���������ũҩ������Һ����ΪCuSO4��Ca(OH)2��I���������Ƽ�����IΪCaCO3��I���ɵ�J��ת����K������K��Ca(OH)2��F��CuSO4��J�� CaO��CaCO3�ֽ����һ������CO2����B��CO2��B��C��Ԫ�������ͬ������C��һ����̼��AΪ��ɫ���壬һ����̼��D��Ӧ������A�Ͷ�����̼������D������ͭ��A��ͭ��CuO��E������CuSO4������E�����ᡣ
��1������F�Ļ�ѧʽ��CuSO4������J�������ƣ����׳���ʯ�ң�
��2����Ӧ����̼��Ƹ��·ֽ���������ƺͶ�����̼��������Ӧ����Ϊ�ֽⷴӦ��
��3����Ӧ�۵Ļ�ѧ����ʽΪ![]() ��
��
��4��CO2��CO�Ļ����3.6g���������ȵ�CuO���ʳ�ַ�Ӧ����CO��CuO��Ӧ��������CO2������������Һ�г�����գ��������������Һ������Ϊ84.4�ˣ���ԭ�к����ɵ�CO2���У�84.4g�C80g=4.4g��CO��CuO��Ӧʱһ��CO���ȡCuO�е�һ��O����CO2�����Ի���������ص�����������Ԫ�ص�������4.4g�C3.6g=0.8g����һ����̼������Ϊ![]() �����������غ㶨���У�
�����������غ㶨���У�  ��
��![]() �����
�����![]() =1.4g��
=1.4g��

����Ŀ������ѡ�õij����Լ���ʵ���������ȷ����(������Ϊ����)
ѡ�� | ���� | ����ѡ�õ��Լ��Ͳ��� |
A | NaCl����(Na2CO3)���� | �����������ᣬ��������ַ�Ӧ�������ᾧ |
B | KCl����(KClO3)���� | ����������MnO2������ |
C | CO2(CO) | ͨ��������������ȼ |
D | NaNO3��Һ(NaOH��Һ) | ��������CuSO4��Һ������ |
A. AB. BC. CD. D