��Ŀ����
�������������������������������Ҫ�Ĵ�������Ⱦ��ҹ�����ʮ�����ӻ��������������������̨ÿ�조�������š���Ҫ����ȫ��42���ص���п��������ձ������������ǹ�ע�Լ����������Ŀǰ���йز��Ÿ���S02��Br2��H2O�Ķ�����Ӧ���ⶨ������SO2�ĺ�����������һԭ��ͨ���ڵ����з�����Ӧʱ�����ı仯������ȷ�ⶨ������SO2�ĺ�����ijУ����С��Ϊ�ⶨУ����������SO2�ĺ�����ijУ����С��Ϊ�ⶨУ����������SO2������������ĺ���������������Ӧԭ�����������װ�á�
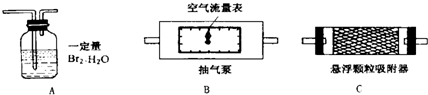
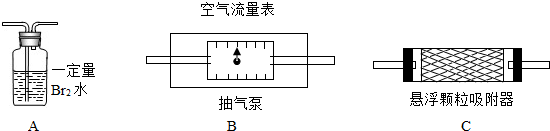
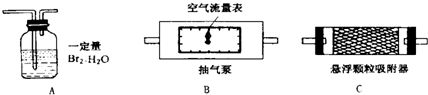
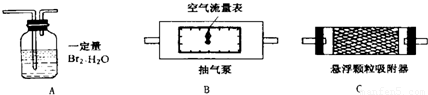
1��������������ȷ�ⶨ�������õĿ����������ⶨ����������S02����ʱ������װ�����ӵ�˳����(�����) ��
2��Ϊȷ�ⶨSO2�ĺ�����ʵ��ʱ���۲쵽A�� ʱ��Ӧ�����رճ����á�
3����Ҫ�ⶨ��������������ʱ�ĺ�������Ҫ���ʵ��ʱ�����������⣬��Ҫ�� (�����)����ʵ��ǰB����������ʵ���B����������ʵ��ǰC����������ʵ���C������
4������С���һλͬѧ��NaOH����Br2��H2O�ⶨSO2�ĺ���ʱ�����ֲⶨ�������ƫ�ߣ���ԭ���� ��
5��ij����������SO2������Դ��Ҫ�� (�����)�ٻ�ɽ��������ֲ��Ĺ�����ã���ú��ʯ�͵�ȼ�գ���ѧУ��ѧʵ��
6�����ݱ�����ʵ�飬������������������Ϳ�����SO2����������������������������� ��(��ʾ������һ���ۺ��Ժ�ǿ����ϵʵ�ʵ�ʵ���⣬Ҫ�������������˼·��Ū��Ҫ�ﵽ��Ŀ�ģ��������ѧ���Ļ�ѧʵ���֪ʶȥ���)��
BCA��CAB 2������ɫ�� 3���ۢܣ�4��NaOH��Һ��Ҫ���տ����е�CO2�����ʣ�
5���ۣ� 6���ı�ȼ�Ͻṹ����ǿβ����������ǿ�̻��������������֣���ǿ���ع����ȡ�