��Ŀ����
����Ŀ�������нϳ��ĺ����ߣ�����������ṩ�˷ḻ����Դ��������ѧ��Դ�������Դ��������Դ�Ͷ�����Դ�ȣ�
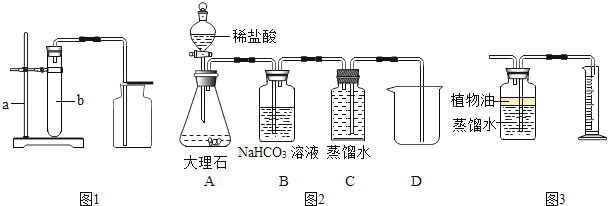
��1���Ӻ�ˮ����ȡ�Ȼ�þʱ�������ˡ���ˮ![]() ������þ
������þ![]() �Ȼ�þ����ת�����̣��˹����н�������þ�Ӻ�ˮ�з�������IJ��������� ��
�Ȼ�þ����ת�����̣��˹����н�������þ�Ӻ�ˮ�з�������IJ��������� ��
��2����ѧ�ҷ��ۺ�������Ŵ����ġ���ȼ������������Ҫ���м���ˮ���������ȼ�գ���һ��δ��������Դ��д�������ڿ����г���ȫȼ�յĻ�ѧ����ʽ ��
��3���������������ڱ�����������_______ ��
A.���������������뺣��
B.�غ�����������ˮֱ�����뺣��
C.��ǿ����������������������Ⱦ�ļ���ˮƽ��
���𰸡�
��1������
��2��CH4+2O2![]() CO2+2H2O
CO2+2H2O
��3��C
����������1��������þ�Dz����Թ��壬����ʹ�ù��˵ķ����Ӻ�ˮ�з��������������ˣ�
��2��������������ȼ�����ɶ�����̼��ˮ�����CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
��3��A�����������������뺣�л���ɺ�ˮ��Ⱦ������
B���غ�����������ˮֱ�����뺣�У�����ɺ�ˮ��Ⱦ������
C����ǿ�����������������Ⱦ�ļ���ˮƽ�����Ա�����������ȷ��
��ѡC��
�����㾫����������Ĺؼ�����������˲�����ע����������֪ʶ�����չ��˲���ע�������һ���������͡������������˺���Һ��Ȼ���ǵĿ���ԭ����:�ٳн���Һ���ձ����ɾ����㵹Һ��ʱҺ�������ֽ��Ե����ֽ�����Լ���ˮ��Դ����Ⱦ����ε����⣬�˽�ˮ��Ⱦ:A��ˮ��Ⱦ���ҵ�����ϡ�����������Һ����������ũҩ�����ʵIJ�����ʩ��������ˮ�������ŷ�B����ֹˮ��Ⱦ����ҵ����Ҫ����������ŷš��ᳫ���ŷţ�������ˮҪ���д�������ŷš��ᳫ���ŷţ�����ʩ��ũҩ�����ʣ��ᳫʹ��ũ�ҷʣ���ǿˮ�ʼ�⣮����ˮ��Դ����Լ��ˮ����ֹˮ����Ⱦ��