��Ŀ����
�յ�����һ��Ⱦɫ�������õĺ�ɫ�ذ�����ɫЬ��ͨ�������յ���ijɷ֣�����2004��4��MSDS�ṩ�����ݣ��յ�����������°����ã�����ʳ�ã�2005��2��23���й����������������棬��ֹ�յ�����ΪʳƷ���Ӽ����±����յ���һ�ŵIJ�����Ϣ��| ��ѧʽ | C16H12N2O |
| ��� | ����ɫ�����ɫƬ״���� |
| �ܽ�� | ��ˮ�У���0.01g/100mL�������У������������ |
| �۵� | 404��406�� |
| ���� | 475�� |
| �°�ԭ�� | �������ڷֽ��һ���ж����л�������� |
��1���յ���һ���к���______Ԫ�أ�����Է�������Ϊ______�����е�Ԫ�ص���������Ϊ______��
��2�������ϱ���������Ϣ���յ���һ�ž���______�������ʣ�д��1�����ɣ���
��3���յ���һ�ŶԶ�����������°����ã���Ҫ����Ϊ�����������ڷ�����______�仯��
���𰸡��������������ʵĻ�ѧʽ���������ʣ���Է��������Լ���Ԫ�ص���������= ×100%���з�������⣮
×100%���з�������⣮
����⣺��1�������յ���1�ŵĻ�ѧʽC16H12N2O����֪�յ�����C��H��N��O����Ԫ����ɣ�������Է��ӵ�����Ϊ��ɷ��ӵĸ�ԭ�ӵ����ԭ������֮�ͣ��յ���1�ŵ���Է�����Ϊ����12×16+1×12+14×2+16=248�����е�Ԫ�ص���������=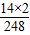 ×100%=11.3%�����C��H��N��O��248��11.3%��
×100%=11.3%�����C��H��N��O��248��11.3%��
��2�����ʲ���Ҫ������ѧ�仯�ͱ��ֳ��������ʣ������������ʣ��������ʰ�������ɫ��״̬����ζ��ζ�����۵㡢�е㡢Ӳ�ȡ��ܶȡ������ԡ������ԡ���չ�ԡ��ܽ��ԡ��ӷ��Եȣ����ݱ��е���Ϣ���յ���1���������ʵ��а���ɫ�����ɫƬ״���塢��ˮ�У���0.01g/100mL�������У�����������ܡ��۵���404��406�桢�е���475�棻
�ʴ�Ϊ������ɫ�����ɫƬ״���壻
��3���յ���1���������ڷֽ��һ���ж����л�������˻�ѧ�仯�����������°����ã��ʴ�Ϊ����ѧ��
���������⿼��ѧ���������ʵĻ�ѧʽ���������ʣ���Է����������з��������������
 ×100%���з�������⣮
×100%���з�������⣮����⣺��1�������յ���1�ŵĻ�ѧʽC16H12N2O����֪�յ�����C��H��N��O����Ԫ����ɣ�������Է��ӵ�����Ϊ��ɷ��ӵĸ�ԭ�ӵ����ԭ������֮�ͣ��յ���1�ŵ���Է�����Ϊ����12×16+1×12+14×2+16=248�����е�Ԫ�ص���������=
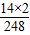 ×100%=11.3%�����C��H��N��O��248��11.3%��
×100%=11.3%�����C��H��N��O��248��11.3%����2�����ʲ���Ҫ������ѧ�仯�ͱ��ֳ��������ʣ������������ʣ��������ʰ�������ɫ��״̬����ζ��ζ�����۵㡢�е㡢Ӳ�ȡ��ܶȡ������ԡ������ԡ���չ�ԡ��ܽ��ԡ��ӷ��Եȣ����ݱ��е���Ϣ���յ���1���������ʵ��а���ɫ�����ɫƬ״���塢��ˮ�У���0.01g/100mL�������У�����������ܡ��۵���404��406�桢�е���475�棻
�ʴ�Ϊ������ɫ�����ɫƬ״���壻
��3���յ���1���������ڷֽ��һ���ж����л�������˻�ѧ�仯�����������°����ã��ʴ�Ϊ����ѧ��
���������⿼��ѧ���������ʵĻ�ѧʽ���������ʣ���Է����������з��������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�յ�����һ��Ⱦɫ�������õĺ�ɫ�ذ�����ɫЬ��ͨ�������յ���ijɷ֣�����2004��4��MSDS�ṩ�����ݣ��յ�����������°����ã�����ʳ�ã�2005��2��23���й����������������棬��ֹ�յ�����ΪʳƷ���Ӽ����±����յ���һ�ŵIJ�����Ϣ��
| ��ѧʽ | C16H12N2O |
| ��� | ����ɫ�����ɫƬ״���� |
| �ܽ�� | ��ˮ�У���0.01g/100mL�������У������������ |
| �۵� | 404��406�� |
| ���� | 475�� |
| �°�ԭ�� | �������ڷֽ��һ���ж����л�������� |
��1���յ���һ���к���________Ԫ�أ�����Է�������Ϊ________�����е�Ԫ�ص���������Ϊ________��
��2�������ϱ���������Ϣ���յ���һ�ž���________�������ʣ�д��1�����ɣ���
��3���յ���һ�ŶԶ�����������°����ã���Ҫ����Ϊ�����������ڷ�����________�仯��

