��Ŀ����
ij�о���ѧϰС��ѧϰ�˹�ҵ�������Ƽ����ԭ����֪�����·�Ӧ��
NaCl �� NH3 �� CO2 �� H2O = NaHCO3���� NH4Cl ��
��������⡿�ܷ���ʵ����ģ�⡰�����Ƽ����ȡNaHCO3�Ĺ����أ�
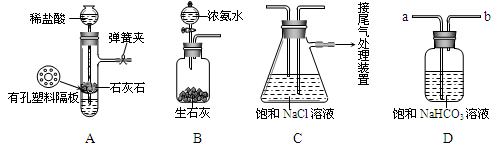
��ʵ����֤������ͼ�Ǹ�ѧϰС�����ģ��ʵ��ʱ���õ��IJ�����Ҫװ�ú�ҩƷ��
��ش��������⣺
�� ����Aװ�������Եķ����ǣ�����������©�����������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ������Թ��ڵ�ˮ�棬ֹͣ��ˮ���� ��˵��װ�ò�©����
�Ƹ�ʵ������Bװ����ȡ�������� ���ѧʽ����Bװ����ʢװŨ��ˮ����������Ϊ ����ʵ����ʹ�ø��������ŵ��� ��
��D��������װ��A��װ��C֮������徻��װ�ã��������� ����a��b����D�������dz�ȥHCl���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ��ʱ����NaCl��Һ��ͨ��϶��NH3����Һ�Լ��ԣ�����ͨ��������CO2����ԭ���� ����д��ţ���
�� ʹCO2���ױ����� �� NH3��CO2������ȡ �� CO2���ܶȱ�NH3��
���� �ķ��������ɵ�NaHCO3����ӻ�����з��������
���ó����ۡ����á������Ƽ����ʵ���ҿ�����ȡNaHCO3 ��
NaCl �� NH3 �� CO2 �� H2O = NaHCO3���� NH4Cl ��
��������⡿�ܷ���ʵ����ģ�⡰�����Ƽ����ȡNaHCO3�Ĺ����أ�
��ʵ����֤������ͼ�Ǹ�ѧϰС�����ģ��ʵ��ʱ���õ��IJ�����Ҫװ�ú�ҩƷ��

��ش��������⣺
�� ����Aװ�������Եķ����ǣ�����������©�����������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ������Թ��ڵ�ˮ�棬ֹͣ��ˮ���� ��˵��װ�ò�©����
�Ƹ�ʵ������Bװ����ȡ�������� ���ѧʽ����Bװ����ʢװŨ��ˮ����������Ϊ ����ʵ����ʹ�ø��������ŵ��� ��
��D��������װ��A��װ��C֮������徻��װ�ã��������� ����a��b����D�������dz�ȥHCl���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ��ʱ����NaCl��Һ��ͨ��϶��NH3����Һ�Լ��ԣ�����ͨ��������CO2����ԭ���� ����д��ţ���
�� ʹCO2���ױ����� �� NH3��CO2������ȡ �� CO2���ܶȱ�NH3��
���� �ķ��������ɵ�NaHCO3����ӻ�����з��������
���ó����ۡ����á������Ƽ����ʵ���ҿ�����ȡNaHCO3 ��
��1��©�������Թ��е�Һ��ˮ�����ֲ����©���е�Һ��ˮ���治���½� ��2��NH3
��Һ©�� ���ڿ�������IJ������ʺͲ��� ��3��a NaHCO3�� HCl =" NaCl" �� H2O �� CO2�� ��4�� �� ��5������
��Һ©�� ���ڿ�������IJ������ʺͲ��� ��3��a NaHCO3�� HCl =" NaCl" �� H2O �� CO2�� ��4�� �� ��5������
�����������1��ע��һ������ˮ���Թ������屻ѹ������ѹǿ����©����Һ�岻���������Թܣ�ʹ����©�������Һ�����Һ������Һ���ֲ��䣬˵��װ�����������ã�
��2����ʯ�ҷ���ʹŨ��ˮ�ӷ�������������������ѧ������֪�Ƿ�Һ©����B�ǹ�Һ�����ͣ��÷�Һ©�����ڿ�������IJ������ʺͲ�����
��3��Ϊ�����������Ŀ��Ӧʹ����ͨ����Һ����������Ӧ��aͨ����Һ��ͨ����Һװ��D��ʢ�ŵ�̼��������Һ������������е�HCl������Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���Ӷ���ȥ���������е�HCl����Ӧ�Ļ�ѧ����ʽΪ��NaHCO3+HCl�TNaCl+H2O+CO2����
��4����ͨ�백����������ˮ�γɳʼ��Եİ�ˮ�����������̼��ˮ���ɵ�̼�ᷢ����Ӧ������кͣ��������ڶ�����̼��������գ�
��5���ѹ�����Һ�����γɵĻ�����й����������ķ���Ϊ���ˡ�ͨ�����ˣ��ɰѻ�����Һ�е�̼�����ƾ�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ