��Ŀ����
��3�֣��������ܴ�ˮ���ܴ���ˮ���٣���ˮ���ܽ��˺ܶ����ʣ��ֿ����̣����˺�H2O�⣬�����д�����Na+ ��Ca2+��Cl-��Mg2+��SO42- �ȡ��Ժ�ˮΪԭ����ȡʳ�εĹ����������£�
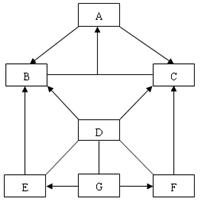
��1��ͼ�Т��� �أ������������ȴ������
��2�����ݺ�ˮɹ�ε�ԭ��������˵���в���ȷ���� ��
A�� �õ��Ĵ����Ǵ������Ȼ���
B�� �ڢ��У���ˮ��ˮ����������
C�� �ڢ��У���ˮ���Ȼ��Ƶ�����������
D�� ��ˮ������ˮ�أ���ˮ�ijɷֻ�������
��3��������������������ͼ��ĸҺ�������������� ��
A�� Ca2+��Mg2+��SO42-�� B�� Na+ ��Ca2+��Cl-��Mg2+��SO42-��H2O��
C�� Ca2+��Mg2+��SO42-��H2O�� D�� Na+ ��Ca2+��Cl-��Mg2+��SO42-��
��1������ ��2��AC ��3��D
���������������1���Ȼ��Ƶ��ܽ�����¶ȵ����߶��仯���ʴӺ�ˮ����ȡ�Ȼ���Ӧ��ȡ�����ᾧ�ķ���
��2��A���õ��Ĵ�������Ҫ���Ȼ��ƵĻ�������B���ڢ��У��Dz�������ˮ�ֵķ��������Ժ�ˮ��ˮ���������٣���ȷ��C���ڢ��У�������ˮ�ֵĹ����У���ˮ���Ȼ��Ƶ�����Ӧһֱ���䣬����D����ˮ������ˮ�أ���ˮ�ijɷֻ������䣬��ȷ����ѡA��C
��3����Ϊ��ˮ���ܽ��˺ܶ����ʣ��ֿ����̣����˺�H2O�⣬�����д�����Na+ ��Ca2+��Cl-��Mg2+��SO42- �ȣ���ͨ������ˮ�֣�ֻ�Ǵֵ��Ȼ��ƽᾧ����������ĸҺ��������������Ȼ�У�Na+ ��Ca2+��Cl-��Mg2+��SO42-�ȣ�ѡD
���㣺�Ȼ��Ƶ��ܽ�����¶ȵı仯����������ᾧ

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��ᡢ��κ��������֪ʶ�dz��л�ѧ��Ҫ�Ļ���֪ʶ��������ͬѧ�Ƕ����֪ʶ����̽���Ĺ��̣�
��1����ͼ������ж�������Ԫ�أ���ͼ������Ԫ�����ڱ��е�һЩ��Ϣ�����ж�ͼ����Ϣ�������Ӧ�ô�������� ����
��A��ʾ��ԭ�Ӻ��к���һ������ ��B��Ԫ�صķ��� ��C��Ԫ�ص����� ��D��ԭ�ӵ�����
��2�����ᣬ������������ᶼ�����ƵĻ�ѧ���ʣ��磺���Ƕ���ʹ���ָʾ����ɫ�����Ƕ��� ����Ũ�������� �����ʿ��������������Ũ����ܸ��ﰱ������Ϊ�����������ᷴӦ����������泥��÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3���������ơ��Ȼ��ơ�����ͭ�����ʵ�ˮ��Һ�ܵ��磬�������ǵĹ���ȱ���ܵ��磬����Ϊʲô��
��4��ˮ����Ҫ����������þ��̼�����ɵĻ���ͬѧ�Ƿֱ���ϡ�����ϡ��������ȥˮ����������ϡ����ܿ���ܽ�ˮ����ȥ��������ϡ�����ˮ��ʱ����Ӧ����ֹͣ������Ϊϡ����ܽ�ˮ����ȫ������ԭ������ ��
��5��Ϊ�˲ⶨˮ���е�̼��Ƶĺ�����ͬѧ�dz�ȡ10.0gˮ�������ձ��У���4�ν�ϡ�������ձ��м��룬����������±���
| | δ��ϡ����ʱ | ��һ�μ���10.0gϡ���� | �ڶ��μ���10.0gϡ���� | �����μ���10.0gϡ���� | ���Ĵμ���10.0gϡ���� |
| �ձ������������� | 10.0g | 18.9g | 27.8g | 36.7g | 46.7g |