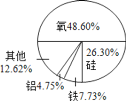
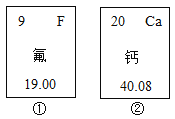
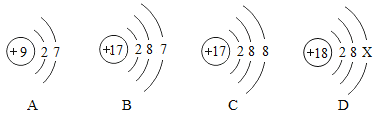
��Ŀ����
����Ŀ�����о���ͼ�Ļ�������ʱ��ijͬѧ��֤����ϡ���������������Һ��Ϻ���Ȼû�з������Եı仯����ȷʵ�����˻�ѧ��Ӧ������������һ���������̽����
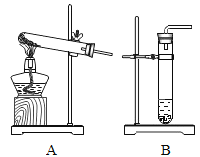
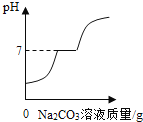
(1)����һ���ⶨϡ���������������Һ���ǰ���pH(����)���ⶨijNaOH��Һ��pH����7��Ȼ��һ������ϡ��������NaOH��Һ�У���Ͼ��Ⱥ����pHС��7��
���ۣ�ϡ�����NaOH��Һ�����˻�ѧ��Ӧ������___�������÷�Ӧ�Ļ�ѧ����ʽΪ___��
(2)���������������ָʾ����
��С��˵��������ͼ1��ʾʵ�鲽�����ʵ�飬�۲쵽____ʱ��ϡ�����NaOH��Һǡ����ȫ��Ӧ��
��С��˵��ʵ�鿪ʼ����Ҫ�μ���ɫ��̪��Һ����ʵ���������Һ�еμӼ�����ɫ��̪��Һ������Һ��ɫû�б仯��������ǡ����ȫ��Ӧ��
������������Ϊ���ǵ�˵����____��˵������ȷ��������____��
������ʵ�飩�������һ��ʵ�飬̽�������ձ��е���Һ�Ƿ������ԣ�
ʵ����� | ʵ������ | ʵ����� |
ȡ��Ӧ�������1-2mL���Թ��У����� ____���� | ____ | ��Һ������ |
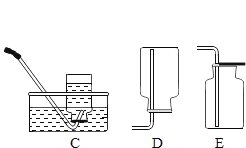
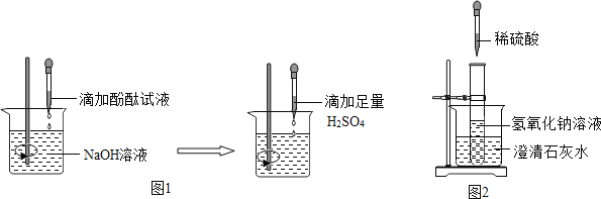
(3)���¶ȱ仯��Ϊ�˽�һ����ȡϡ����������������Һȷʵ�����˷�Ӧ��֤�ݣ������кͷ�Ӧ��____(�������������������������������仯��)�ķ�Ӧ��С����ͼ2װ�ý���ʵ�飬�ɹ۲쵽�ձ��еij���ʯ��ˮ____����ԭ����____��
���𰸡�ϡH2SO4 2NaOH+H2SO4=Na2SO4+2H2O ��Һ�ɺ�ɫǡ�ñ�Ϊ��ɫ С�� ���ϡ���������������ɫ��̪��Һ����ҺҲ����ɫ п�� �����ݲ��� ���� ����� Ca(OH)2���ܽ�����¶����߶���С������ʯ��ˮ����ǣ�˵��ϡ�������������Ʒ�����Ӧ���ų���������Һ�¶�����
��������
(1)����һ�����ۣ�ϡH2SO4��NaOH��Һȷʵ�����˻�ѧ��Ӧ����ΪpH��7����ϡH2SO4������H2SO4��NaOH��Ӧ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+H2SO4=Na2SO4+2H2O�����ϡ���2NaOH+H2SO4=Na2SO4+2H2O��
(2)����������С��˵������ͼʾʵ�鲽�����ʵ�飬�۲쵽������������Һ�����̪��Һ����Һ���ɫ���ټ�������ϡ���ᣬ�۲쵽��ɫ��Һ�ɺ�ɫǡ�ñ�Ϊ��ɫ��ϡ�����NaOH��Һǡ����ȫ��Ӧ�������Һ�ɺ�ɫǡ�ñ�Ϊ��ɫ��
��С�ĵ�˵������ȷ�������ǣ���Һ�����ԣ���ɫ��̪��ҺҲ����ɫ�����ʵ�飺���Թ�ȡ����Һ1-2mL������2-3����ɫʯ����Һ������Һ���ɫ��ʵ����ۣ���Һ�����ԣ����С�ģ����ϡ���������������ɫ��̪��Һ����ҺҲ����ɫ��
������ʵ�顿
ʵ����� | ʵ������ | ʵ����� |
ȡ��Ӧ�����Һ1-2ml���Թ��У����� п��(�� ��ɫʯ����Һ��)����(����������Ҳ��) | �����ݲ���(��Һ���ɫ)(����������Ҳ��) | ��Һ������ |
���п���������ݲ�����
(3)��������Ϊ�˽�һ����ȡϡH2SO4��NaOH��Һȷʵ�����˻�ѧ��Ӧ��֤�ݣ������кͷ�Ӧ�Ƿ��ȵķ�Ӧ��Ca(OH)2���ܽ�����¶����߶���С������ʯ��ˮ����ǣ�˵��ϡ�������������Ʒ�����Ӧ���ų���������Һ�¶����ߣ�������ȣ�����ǣ�Ca(OH)2���ܽ�����¶����߶���С������ʯ��ˮ����ǣ�˵��ϡ�������������Ʒ�����Ӧ���ų���������Һ�¶����ߡ�

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�����Ŀ����У��ȤС�����ľ̿��һ����̼������ͭ��Ӧ��̽��ʵ�飬�ش�������⡣
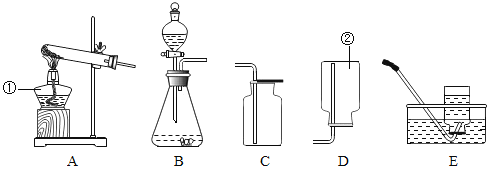
ʵ��һ:��ͼʵ�����̼��ԭ����ͭʵ��,
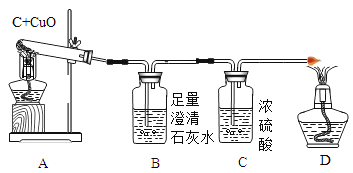
���������ϣ�
(1)������ͭ(Cu2O) �Ǻ�ɫ���壬����ϡ���ᷢ�����·�Ӧ: ![]() ��
��
��������⣩��֪ʵ����Թ��еĺ�ɫ���庬�е���ͭ���Ƿ���������ͭ(Cu2O) ��?
��ʵ����֤��ȡ�Թ��еĺ�ɫ�����������Թ��У�����_________��Һ���Թ��г���__________��֤��������ȷʵ����Cu2O��
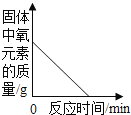
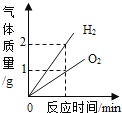
(2)�Ӷ�������Ƕ��жϲⶨ�����е��ĸ�����:����ֻ��CO2���ɣ�������m4- m3______m1-m2(ѡ�>������<����=��)��A�з�����Ӧ�Ļ�ѧ����ʽ��_____________��ʵ�������ɵ����廹��CO����������CO�Ŀ���ԭ��(дһ��)__________________��
��Ӧǰ������ | ��Ӧ������� | |
װ��A | m1 | m2 |
װ��B | m3 | m4 |
��ʵ�����ʱ�Ȱε�A���Թܿڵ���Ƥ������ֹͣ���Թܼ��ȣ���Ŀ����________________��
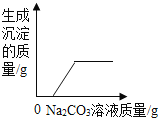
ʵ���:ͬѧ�Ƕ���һ������ͭ��Ʒ������ͭ�ĺ������вⶨ�����dz�ȡ��20g��������ͭ��Ʒ����ͼ1��ʾ��װ�ý���ʵ�飬����Ӧ��ȫ(���ʲ����뷴Ӧ��ʯ��ˮ����)���ⶨ�IJ���������ͼ2��ʾ:
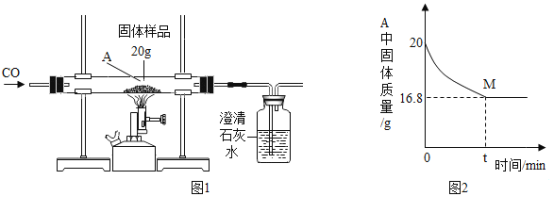
(1)����ʯ��ˮ��������________________��
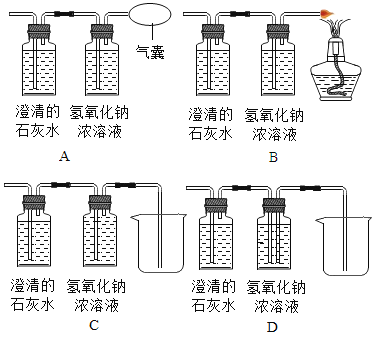
(2)��ͬѧ��Ϊͼ1װ�ò������ƣ�Ϊ�˷�ֹβ���Կ�������Ⱦ��Ӧ�ð�ͼ1װ���е�ʢ�г���ʯ��ˮ��װ�û�������____________װ��(����ĸ���)��
(3)�ò��Ⱥ��װ�ý��д�ʵ�飬����ǰ��ͨһ���һ����̼��Ŀ����________________��
(4)ͼ2��M��ĺ�����________________��
(5)����Ʒ������ͭ����������Ϊ________________��
(6)��ʵ���������������䣬ֻ��CO��ΪH2����ʵ�飬��������ͭ��Ʒ������ͭ����������Ϊ_________(���ͬ������ͬ��)��