��Ŀ����
����Ŀ����1��Ŀǰ���������Ⱦָ������ĿΪ______��һ����̼�����������������������ͳ����ȣ�����ʱ���������ؾ����������ˮ��Ŀ����______��
��2�������е���Դ�ṹ��������ȼúΪ�������ڵ���Ȼ��ȫ�����У�ú��______����Ȼ������Ϊ��ʯȼ�ϣ���Ȼ������Ҫ�ɷ���ȫȼ�յĻ�ѧ����ʽΪ______��
��3��ˮ������֮Դ��ijѧУ��ֱ��ˮ����������ͼ��ʾ��
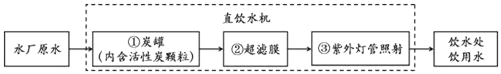
̿���л���̿��������_______������Ĥ��������______������ƹ������������______��
���𰸡��������� �ܽ�����տ���������� ʯ�� CH4+2O2![]() CO2+2H2O ����ɫ�ء���ζ�� ���˳�ȥ������ˮ������ ɱ������
CO2+2H2O ����ɫ�ء���ζ�� ���˳�ȥ������ˮ������ ɱ������
��������
��1��������Ⱦָ������ĿΪ��������һ����̼�����������������������ͳ����ȣ�����ʱ���������ؾ����������ˮ��Ŀ�����ܽ�����տ���������
��2��ú��ʯ�͡���Ȼ������Ϊ��ʯȼ�ϣ���Ȼ����Ҫ�ɷ��Ǽ��飬����ȼ������ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
��3������̿�ܹ�����ɫ�ء���ζ�ȣ�����Ĥ���Թ��˳�ȥ������ˮ�����ʣ������߿���ɱ����������������ƹ������������ɱ��������

����Ŀ����ʯͷֽ����һ�ֽ���ֽ�ź�����֮������Ͳ��ϣ���Ҫ�ɷ�Ϊ̼��ƣ���Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ 50g ��ֽ��Ʒ���ֱ��� 5 ֻ�ձ��н�����ʵ�飬ʵ�����ݼ��±�������ֽ�������ɷּȲ�����ˮ��Ҳ����ˮ��Ӧ����
�ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
����ϡ���������/g | 20 | 40 | 60 | 80 | 100 |
��ַ�Ӧ���������������/g | 0.88 | 1.76 | 2.44 | 3.52 | 3.52 |
��1��10g ��Ʒ������ϡ���ᷴӦ�����������__�ˡ�
��2���ձ�__����ţ��м�¼��ʵ�����������Դ���������__��
��3������������������������_____����д��������̣�