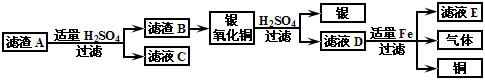
��Ŀ����
����ͭ��Ũ���ᡢϡ���ᷴӦ�ķ���ʽ���£�
Cu��4HNO3(Ũ)��Cu(NO3)2��2NO2����2H2O ��NO��NO2���嶼�ǿ�������Ⱦ�
3Cu��8HNO3(ϡ)��3Cu(NO3)2��2NO����4H2O (NO������O2��������NO2)
��ҵ�ϳ���ͭмΪԭ����ȡ����ͭ����ʵ�������У����Ũ������ˮϡ�͡��Իش�
��1��Ϊʲô��ϡ���������Ũ��� ��
��2���Ӿ���Ч��ͻ����Ƕȿ��ǡ������ȡ����ͭ�������˷�����
ijͬѧ���룺�Ƚ�ͭм��������Ӧת��Ϊ��ɫ���壬���������ܽ⣻��д���йػ�
ѧ����ʽΪ�� �� ��
����Ϊ�����������Ƿ���ԣ��������ɣ� ��
Cu��4HNO3(Ũ)��Cu(NO3)2��2NO2����2H2O ��NO��NO2���嶼�ǿ�������Ⱦ�
3Cu��8HNO3(ϡ)��3Cu(NO3)2��2NO����4H2O (NO������O2��������NO2)
��ҵ�ϳ���ͭмΪԭ����ȡ����ͭ����ʵ�������У����Ũ������ˮϡ�͡��Իش�
��1��Ϊʲô��ϡ���������Ũ��� ��
��2���Ӿ���Ч��ͻ����Ƕȿ��ǡ������ȡ����ͭ�������˷�����
ijͬѧ���룺�Ƚ�ͭм��������Ӧת��Ϊ��ɫ���壬���������ܽ⣻��д���йػ�
ѧ����ʽΪ�� �� ��
����Ϊ�����������Ƿ���ԣ��������ɣ� ��
��1��ϡ������������ͭ��ת���ʸߣ�������Ⱦ�����������١������һ�㼴���֣�
��2��2Cu + O2 2CuO����CuO��+ 2HNO3=== Cu(NO3)2 + H2O
2CuO����CuO��+ 2HNO3=== Cu(NO3)2 + H2O
���ԣ���ӦЧ�ʸߣ���������Ⱦ���������塣�����һ�㼴���֣�
��2��2Cu + O2
 2CuO����CuO��+ 2HNO3=== Cu(NO3)2 + H2O
2CuO����CuO��+ 2HNO3=== Cu(NO3)2 + H2O���ԣ���ӦЧ�ʸߣ���������Ⱦ���������塣�����һ�㼴���֣�
��1����ͭм��Ũ���ᡢϡ����Ϊԭ����ȡ����ͭ�����˶���������һ���������������к�������Ⱦ�˻������۲췽��ʽ��֪��Cu��4HNO3��Ũ����3Cu��8HNO3��ϡ�����Ʊ�������������ͭʱ���ĵ�ϡ�����Ũ�����٣���Լ��ԭ�ϣ�
��2���Ӿ���Ч��ͻ����Ƕȿ��ǡ������ȡ����ͭ�������˷�����ʹ��ͭ���Ȱ�ͭ�������ڼ��ȵ���������������ͭ������������ͭ��ϡ���ᷴӦ��������ͭ��ˮ����������Լ��ԭ�ϣ��ֱ�������Ⱦ��
�ʴ�Ϊ����1���Ʊ�������������ͭʱ���ĵ�ϡ�����Ũ�����٣�����ͬ������ϡ�����Ũ��������������ͭ�ࣩ��
��2��2Cu+O2
 2CuO CuO+2HNO3=Cu��NO3��2+H2O��
2CuO CuO+2HNO3=Cu��NO3��2+H2O�����ԣ���ӦЧ�ʸߣ���������Ⱦ���������壮

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ