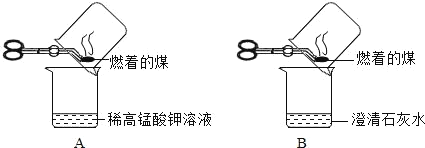
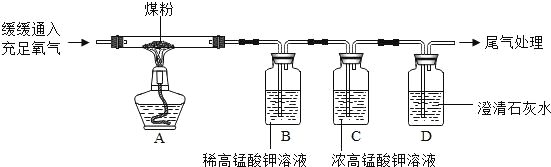
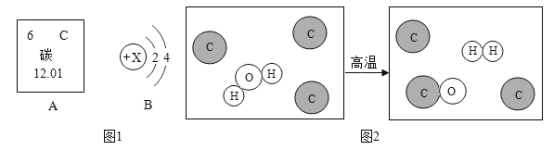
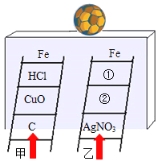
��Ŀ����
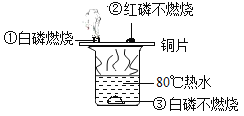
����Ŀ����Դ�뻷��������ע�Ľ��㡣��ͼ��ij̫���ܵ��ʾ��ͼ,�ش���������:
(1)ͼ����������,���еĽ���Ԫ����_____(��1�ּ���)�����е��л��ϳɲ�����_______��
(2)̫���ܵ���жദʹ�úϽ𣬺Ͻ��봿������ȣ��������������ܣ���:____(д��1������)��
(3)ͭ��¶�ڳ�ʪ�Ŀ����л�����.������ͭ[Cu2(OH)2CO3]������ͭ��O2��H2O��_____(�ѧʽ)��Ӧ�IJ��
(4)�ƹ�ʹ��̫���ܵ�ص�������___________(д��1�㼴��)��
���𰸡�ͭ���������� ���� ����ʴ�Ժ� CO2 ���ٶԻ�������Ⱦ
��������
��1��ͼ�����������У����еĽ���Ԫ����ͭ��������������ͭ������������
���е����������л��ϳɲ��ϣ��������ϡ�
��2���Ͻ���ŵ��п���ʴ�Ժõȣ������ʴ�Ժã��������ɣ���
��3�����������غ㶨�ɿ��жϷ�Ӧ�ﺬ�к�̼Ԫ�ص����ʣ��������к�̼������ΪCO2������CO2��
��4���ƹ�ʹ��̫���ܵ�ؿɼ��ٻ�ʯȼ�ϵ�ʹ�ã����ٶԻ�������Ⱦ��������ٶԻ�������Ⱦ���������ɣ���

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�����Ŀ��ij������ȤС��Ϊ̽��һ���õ���Ƭ����Ԫ�ص���������,����ȡ8 g����Ƭ��Ʒ�����ձ���,�����μ���һ����������ͬŨ�ȵ�ϡ���ᡣ��������������,��ش����⡣
ʵ����� | 1 | 2 | 3 | 4 | 5 |
ÿ�μ���ϡ���������/g | 50.0 | 100.0 | 100.0 | 100.0 | 100.0 |
���������������/g | 0 | 0.2 | a | 0.6 | 0.7 |
��1��a����ֵΪ_____��
��2����ʽ��������ϡ�����������������_____�����������0.1%����
��3����1��ʵ��û�����������ԭ������ǣ��û�ѧ����ʽ��ʾ��_____��