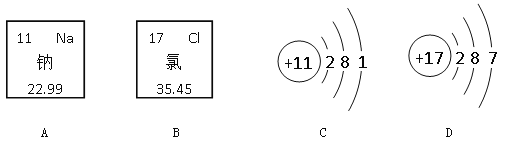
��Ŀ����
����Ŀ��������ϲ���Ի�������õĻ��ȼ����һ���׳ơ�����ƾ��������ʡ���֪������ƾ������þƾ���C2H5OH��������ƺ�ˮ��һ�������Ƴɵġ���������
��1��C2H5OH��C��H��Oԭ�Ӹ�����Ϊ____________��
��2����������ƾ������ȼ��ʱ��ֻ������Ӧ��C2H5OH + 3O2![]() 2CO2 + 3H2O����ȡ������ƾ���30g�����ȼ�պ�����44 g CO2������㡰����ƾ�����C2H5OH�����������Ƕ��٣����������һλС����
2CO2 + 3H2O����ȡ������ƾ���30g�����ȼ�պ�����44 g CO2������㡰����ƾ�����C2H5OH�����������Ƕ��٣����������һλС����
���𰸡���1��2��6��1 ����2��76.7%
��������
���������������1��C2H5OH��C��H��Oԭ�Ӹ�����Ϊ_��ѧʽ��Ԫ�ط������½ǵĽDZ�֮�ȹ�Ϊ2��6��1
��2��������44g CO2���ĵ�C2H5OH������Ϊx
C2H5OH + 3O2![]() 2CO2 + 3H2O
2CO2 + 3H2O
46 88
x 44g
![]()
x=23 g
������ƾ�����C2H5OH������������![]()
�𣺡�����ƾ�����C2H5OH����������Ϊ76.7%��

��ϰ��ϵ�д�
�����Ŀ