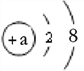��Ŀ����
����Ŀ��ijƷ�Ʋ�����(����ͼ)��ʹ��˵���鲿���������£�

���� �ݡ���Ʒÿ��������������0.1g�������������Ļ�ѧʽ��C4H2FeO4
���������Ρ����ڸ���ԭ��(������ʧѪ��Ӫ������)�����ȱ����ƶѪ����
���÷��������ڷ���
��ͯ��һ��0.1g��һ��1-3�Σ�
���ˣ�һ��0.2g��һ��3-4��
(1)��������������_____��Ԫ�أ���������������Է�������Ϊ______��
(2)������������̼����Ԫ�ص�������Ϊ_____����������������Ԫ�ص���������Ϊ_____��
(3)����ͯÿ�հ��������������ò�������һ��Ӧ���øò�����������Ϊ_____g
���𰸡� 4 170 24��1 32.9% 0.3g
����������1�������û�ѧʽ��������ݻ�ѧʽ�ļ���������2�������û�ѧʽ����Ԫ�ص������Ⱥ�ijԪ�ص����������������3��������������Ϣ����Ԫ�ص������������㡣�⣺��1���������������Ļ�ѧʽ��C4H2FeO4��֪�����������к���4��Ԫ�أ���Է�������=12��4+1��2+56+16��4=170����2���������������Ļ�ѧʽ��C4H2FeO4��C��H=48��2=24��1��C4H2FeO4��Fe%=![]() ��100%=32.9%����3������ͯ��һ��0.1g��ÿ�����Σ�һ��Ӧ���øò�����������Ϊ0.1g��3=0.3g��
��100%=32.9%����3������ͯ��һ��0.1g��ÿ�����Σ�һ��Ӧ���øò�����������Ϊ0.1g��3=0.3g��