��Ŀ����
����Ŀ������̼��ƹ㷺Ӧ���������ϡ����ᡢˮ��Ϳ���Լ���ֽ����ҵ���ճ������е�����Ҳ��������Ħ������Ŀǰ����̼��Ƶ�������Ҫ��̼�������������̼���ͼ��
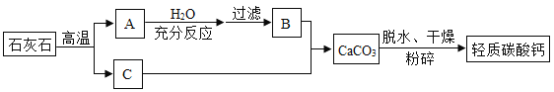
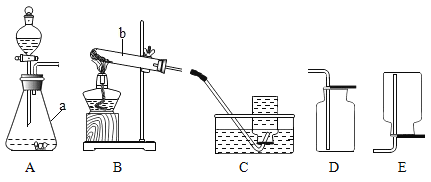
���������̼��Ƶ��������̣��ش��������⣺
��1��ʯ��ʯ��Ҫ�ɷֵĻ�ѧʽΪ________��
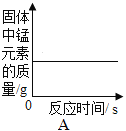
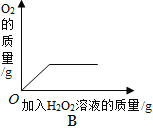
��2����A��B֮�䣬���˲�����Ŀ����________���õ��IJ��������в��������ձ���________�ȣ����в�������������________��
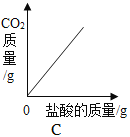
��3��B��C��Ӧ��ѧ����ʽΪ________��
���𰸡�![]() ��ȥ���������� ©�� ����
��ȥ���������� ©�� ���� ![]()
��������
��1��������������̼��Ƶ���������ͼ��֪��A����Ҫ�ɷֺ��������ƣ�C�Ƕ�����̼��B�dz���ʯ��ˮ�����У�ʯ��ʯ��Ҫ�ɷ�Ϊ̼��ƣ���ѧʽΪ![]() ��
��
��2����A��B֮�䣬���˲�����Ŀ���dz�ȥ���������ʣ��õ��IJ��������в��������ձ���©���ȣ����в�������������������
��3��B��C��Ӧ�����������������̼��Ӧ����̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ![]() ��
��

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�����Ŀ��ijͬѧΪ�˲ⶨ��ͭм����п��ͭ�γɵĺϽ���Ʒ��ɣ�ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
ȡ��Ʒ������g�� | 50��0 | 50��0 | 50��0 | 50��0 |
ȡϡ����������g�� | 40��0 | 80��0 | 120��0 | 160��0 |
��������������g�� | 0��4 | 0��8 | 1��0 | 1��0 |
�Լ��㣺
�����ݲ�õ����ݷ�������1����Ʒ�� �������ʣ���ȫ��Ӧ�ˡ�
����ʽ�����ͭм��Ʒ�е�п������������
������ͼ�л�����50��0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
����Ŀ����ͼ��ijͬѧ�Լ���Ƶ�װ�ã��ô�����ƿ�ӽ�ȥƿ�ף���ƿ��һ��Լ8cm��10cm��ƿ����һ������������������A��B�������öƸ���������ֱ���ɣ�������¶ͷ�����ӵ��ߡ��Իش�
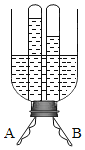
��1�������õ�ԴΪ______�磬��ͼ��֪A��Ϊ______����
��2����A���������Թ��еõ���������_______������ķ�����_____________��
��3������B�������Թ�����������Ϊ8mL������A�������Թ�����������Ϊ______��
��4����ij�ε��ˮ��ʵ���м������������������ƣ�NaOH����Һ��Ŀ����________________�� ����˷ֱ����Դ�������������������������ϲ����������ʵ���������£�
ʱ��/���� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
�����������������cm3�� | 6 | 12 | 20 | 29 | 39 | 49 | 55 | 65 | 75 | 85 |
�����������������cm3�� | 2 | 4 | 7 | 11 | 16 | 21 | 26 | 31 | 36 | 41 |
��ϸ��������ʵ�����ݣ�1��6����������������������������֮�ȴ���2��l�����ܵ�ԭ����__________________��
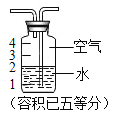
��5����ͼΪˮ�����̵���ʾ��ͼ�����ܴ�ͼ���ܵó���Щ��Ϣ��д����������

��������ɷ���__________________________��
�ڱ仯���ͷ���__________________________��
���۱仯����__________________________��
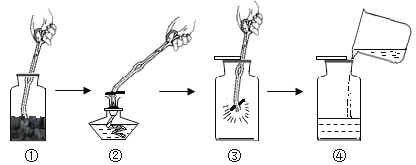
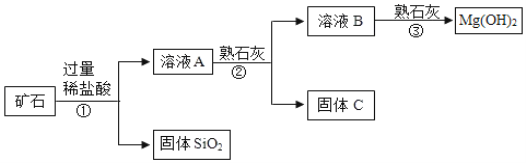
����Ŀ��ij��ʯ��Ҫ�ɷ���MgO����������Fe2O3��CuO��SiO2���ʡ��øÿ�ʯ�Ʊ�Mg(OH)2�Ĺ������̼�ͼ��ͼ��
�ش��������⣺
��1��������з�����Ӧ�ķ���ʽ_____����дһ������
��2����ҺA����������������_____�������ӷ��ţ���
��3����֪���ֽ��������������������γɳ���ʱ��Һ��pH���±���
��Ӧ���� | Fe3+ | Cu2+ | Mg2+ |
��ʼ����ʱ��pH | 1.9 | 4.2 | 9.1 |
��ȫ����ʱ��pH | 3.2 | 6.7 | 11.1 |
����ڼ�����ʯ�ң�������Һ��pHӦΪ��_____��PH��_____������C�������ɷֵĻ�ѧʽΪ_____��_____��
��4��������Ƶ�Mg(OH)2�Ļ�ѧ����ʽΪ_____��