��Ŀ����
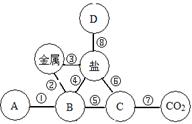
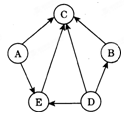
��8�֣�A��B��C��D��E�dz��л�ѧ�г������������ʣ����Ƕ�����һ����ͬ��Ԫ�أ���ͼ��ʾ������֮���ת����ϵ�����У�AΪʳ�ε���Ҫ�ɷ֣�B�к���Ԫ�أ�DΪ����ɫ��Һ��EΪ��ɫ��Һ���������ʵ���Һ��Ϊ��ɫ���ݺ͢�Ϊ�û���Ӧ��������Ϊ���ֽⷴӦ��
��1��д���������ʵĻ�ѧʽ��A ��C ��
��2����Ӧ�ڵ�����Ϊ ��
��3����Ӧ�ݵĻ�ѧ����ʽΪ ��
��4����Ӧ�ߵĻ�ѧ����ʽΪ ��

��1��д���������ʵĻ�ѧʽ��A ��C ��
��2����Ӧ�ڵ�����Ϊ ��
��3����Ӧ�ݵĻ�ѧ����ʽΪ ��
��4����Ӧ�ߵĻ�ѧ����ʽΪ ��
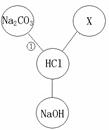
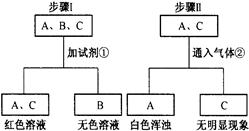
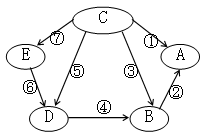
��1��NaCl ��1�֣� HCl��1�֣� ��2��������ɫ������1�֣�
��3��Fe+2HCl===FeCl2+H2����2�֣�
��4��CuO+2HCl===CuCl2+H2O����Cu(OH)2+2HCl===CuCl2+2H2O����3�֣�
��3��Fe+2HCl===FeCl2+H2����2�֣�
��4��CuO+2HCl===CuCl2+H2O����Cu(OH)2+2HCl===CuCl2+2H2O����3�֣�
����Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ����������Ĺؼ����Լ����ʵ����ʺ�����֮��ķ�Ӧ�������жϣ�AΪʳ�ε���Ҫ�ɷ֣���AΪ�Ȼ��ƣ�B�к���Ԫ�أ���BΪ�Ȼ�����C��ת��ΪABDE����CΪ���ᣬD��ת��Ϊ�Ȼ�������D����Ϊ�Ȼ�������E����Ϊ�Ȼ�ͭ�����н��

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ