��Ŀ����
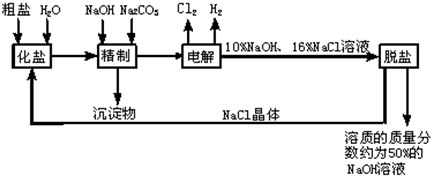
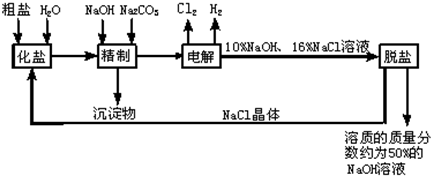
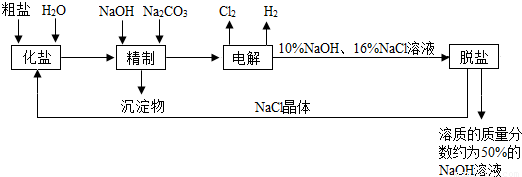
�ȼ��ⱥ��ʳ��ˮ��Һ��ȡNaOH�Ĺ�������ʾ��ͼ��ͼ��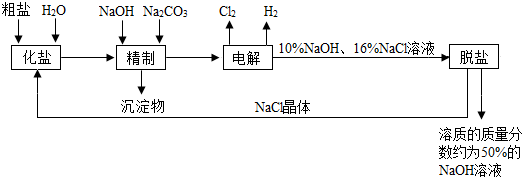
����ͼ�����������գ�
��1�������к��еĽ϶�Ŀ��������ʣ��Ȼ�þ���Ȼ��ơ������Ƶȣ���
��2���ȷ���
����۵���Ԫ��
��2����ҵʳ�κ��н϶����ʣ����ƹ����м�NaOH��������
��3���ڵ������У�������Ӧ�Ļ�ѧ����ʽΪ
������Һ��pH
��4�������������ƺ����ϸߣ��������ӱ��Լ���ȥ��������ӣ��ñ��Լ�������
A��Ba��OH��2 B��Ba��NO3��2 C��BaCl2
��5��Ϊ����Ч�س�ȥ�Ȼ�þ���Ȼ��ơ������ƣ������Լ��ĺ���˳��Ϊ
A���ȼ���NaOH�������Na2CO3���ټ��뱵�Լ�
B���ȼ���NaOH������뱵�Լ����ټ���Na2CO3
C���ȼ��뱵�Լ��������NaOH���ټ���Na2CO3
D���ȼ��뱵�Լ��������Na2CO3���ټ���NaOH
��6�����ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��
��7���ø�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��Cl2��NaOH��Һ��ֽӴ���������NaClO���Ȼ��ƺ�ˮ����д��Cl2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
��������1���ɸ��ݴ��εijɷּ��йػ�ѧʽ���������дҪ����н��
��2����������������Ҫ�dz�ȥþ���ӣ�
��3����ͼ�ɿ�������Ȼ�����Һ���������������غ㶨�ɶ�����ƽ���ɣ���������������ʷ�����ҺpH�ı仯����4�����ӵ�Ҫ��һ�������Լ�����Dz������µ��������ӣ�
��5�����ݳ�ȥ��������̼������ӽ��г�������ȥþ���������������ӽ��г�������ȥ����������ñ����ӳ����������ı�������Ҫ��̼������ӳ�ȥ���з�����
��6�������Ȼ��Ƶ��ܽ�����¶ȱ仯������������з�����
��7����������������Ӧ����������ƽ���ɣ�
��2����������������Ҫ�dz�ȥþ���ӣ�
��3����ͼ�ɿ�������Ȼ�����Һ���������������غ㶨�ɶ�����ƽ���ɣ���������������ʷ�����ҺpH�ı仯����4�����ӵ�Ҫ��һ�������Լ�����Dz������µ��������ӣ�
��5�����ݳ�ȥ��������̼������ӽ��г�������ȥþ���������������ӽ��г�������ȥ����������ñ����ӳ����������ı�������Ҫ��̼������ӳ�ȥ���з�����
��6�������Ȼ��Ƶ��ܽ�����¶ȱ仯������������з�����
��7����������������Ӧ����������ƽ���ɣ�
����⣺��1��������Դ�ں�ˮ����������ɳ�Ȳ��������ʣ�һ������д�ڻ�ѧʽǰ���ʾ������д�����½DZ�ʾ�����ԭ�ӵĸ�����д�����ϽDZ�ʾ���ӵĵ�ɣ���������Ҫд�����ֵ��ұߣ�
�ʴ�Ϊ���ٲ��������ʣ���2Cl2 ��Na+ ��Mg2+ Ca2+ ������H����Na2SO4
��2����������������Ҫ�dz�ȥ�Ȼ�þ�������ķ�ӦΪ��MgCl2+2NaOH=Mg��OH��2��+2NaCl
�ʴ�Ϊ����ȥ�Ȼ�þ��þ���ӣ���
��3����ͼ�ɿ�������Ȼ�����Һ�����������������������������ƣ���ƽ����ʽΪ2NaCl+2H2O
2NaOH+H2��+Cl2 ����Ϊ��ǿ�������������ɣ����Ե�����Һ��pH���ߣ�
�ʴ�Ϊ��2NaCl+2H2O
2NaOH+H2��+Cl2 �������ߣ�
��4����ѡ��Bѡ���Ba��NO3��2������������������ѳ�ȥ������Bѡ���ȷ��
��ѡAC��
��5�����ݳ�ȥ��������̼������ӽ��г�������ȥþ���������������ӽ��г�������ȥ����������ñ����ӳ����������ı�������Ҫ��̼������ӳ�ȥ���������Na2CO3��˳������ڼ��뱵���ӵĺ��漴�ɣ������ĸ�ѡ���֪BCD�������������⣮
�ʴ�Ϊ��BCD��
��6����Ϊ�Ȼ��Ƶ��ܽ�����¶ȱ仯�������Կ��Բ��������ܼ����ᾧ����ȥ���������е��Ȼ��ƣ�
���������
��7�����и����˷�Ӧ��ΪCl2��NaOH��������NaClO���Ȼ��ƺ�ˮ�����������غ㶨����ƽ���ɣ�
�ʴ�Ϊ��2NaOH+Cl2 �TNaClO+NaCl+H2O��
�ʴ�Ϊ���ٲ��������ʣ���2Cl2 ��Na+ ��Mg2+ Ca2+ ������H����Na2SO4
��2����������������Ҫ�dz�ȥ�Ȼ�þ�������ķ�ӦΪ��MgCl2+2NaOH=Mg��OH��2��+2NaCl
�ʴ�Ϊ����ȥ�Ȼ�þ��þ���ӣ���
��3����ͼ�ɿ�������Ȼ�����Һ�����������������������������ƣ���ƽ����ʽΪ2NaCl+2H2O
| ||
�ʴ�Ϊ��2NaCl+2H2O
| ||
��4����ѡ��Bѡ���Ba��NO3��2������������������ѳ�ȥ������Bѡ���ȷ��
��ѡAC��
��5�����ݳ�ȥ��������̼������ӽ��г�������ȥþ���������������ӽ��г�������ȥ����������ñ����ӳ����������ı�������Ҫ��̼������ӳ�ȥ���������Na2CO3��˳������ڼ��뱵���ӵĺ��漴�ɣ������ĸ�ѡ���֪BCD�������������⣮
�ʴ�Ϊ��BCD��
��6����Ϊ�Ȼ��Ƶ��ܽ�����¶ȱ仯�������Կ��Բ��������ܼ����ᾧ����ȥ���������е��Ȼ��ƣ�
���������
��7�����и����˷�Ӧ��ΪCl2��NaOH��������NaClO���Ȼ��ƺ�ˮ�����������غ㶨����ƽ���ɣ�
�ʴ�Ϊ��2NaOH+Cl2 �TNaClO+NaCl+H2O��
������������һ���ۺ������⣬�漰֪ʶ��Ƚ϶࣬Ҫ��ѧ����������֪ʶ�ṹ�ͷ��������������

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ