��Ŀ����
���������Ϊ2013��ȫ����������Ľ��㡣
��1��������Ⱦ����Ҫ�� ���к����壬������Ҫ��Դ�ڻ�ʯȼ�ϵ�ȼ�գ������ķ����ͳ��е� ��
��2������ɫ��ѧ���ĺ����ں����ڷ�Ӧ���̻������У��������ٻ�����ʹ�á������к����ʣ���ֹ�ŷŵ������ж�������ƻ�����Ⱦ��
������ʵ�鲻������ɫ��ѧ�ں����� ������ţ���
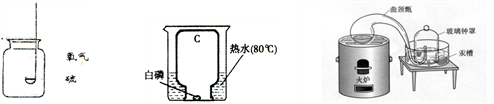
A.��ȼ�� B����ȼ�� C���������ⶨ��������������
����ʵ��Aװ���л�����ĸĽ���ʩ��
��ʵ��C��ʵ�� ������ţ�����ɣ������ʵ�����ɫ����
���������������ɫ��ѧ�ں����� ������ţ���
A��ʵ������˫��ˮ����������ȡ���� B������ú�Ȼ�ʯȼ�ϣ����������Դ
C�������ŷŵ�β����Ϊ��߿��ŷ� D��ֲ������
��1��������Ⱦ����Ҫ�� ���к����壬������Ҫ��Դ�ڻ�ʯȼ�ϵ�ȼ�գ������ķ����ͳ��е� ��
��2������ɫ��ѧ���ĺ����ں����ڷ�Ӧ���̻������У��������ٻ�����ʹ�á������к����ʣ���ֹ�ŷŵ������ж�������ƻ�����Ⱦ��
������ʵ�鲻������ɫ��ѧ�ں����� ������ţ���

A.��ȼ�� B����ȼ�� C���������ⶨ��������������
����ʵ��Aװ���л�����ĸĽ���ʩ��
��ʵ��C��ʵ�� ������ţ�����ɣ������ʵ�����ɫ����
���������������ɫ��ѧ�ں����� ������ţ���
A��ʵ������˫��ˮ����������ȡ���� B������ú�Ȼ�ʯȼ�ϣ����������Դ
C�������ŷŵ�β����Ϊ��߿��ŷ� D��ֲ������
��1���̳����۳��� ����β����2����AC �� B ��ABD
�����������1����������Ⱦ����Ҫ���̳����۳������к����壨����������������һ����̼����������������Ҫ��Դ�ڻ�ʯȼ�ϵ�ȼ�գ������ķ����ͳ��е�����β������2����A������������ȼ�ղ�����Ⱦ�����������������C���������ⶨ��������������������ǿ�ȣ���¯��ȼ�ϲ����ȼ�ղ�����Ⱦ������һ����̼����ѡAC��B������ȼ�ղ��������İ��̣������������������壬��Ӧ���ܱ������������������ײ�����Ⱦ���������ʣ�����ʵ��C��ʵ��B������ţ�����ɣ������ʵ�����ɫ������A��ʵ������˫��ˮ����������ȡ��������Ӧ����Ҫ���ȣ���������ˮ����������ѡA��B������ú�Ȼ�ʯȼ�ϣ����������Դ ���ܼ�С��Ⱦ������������ŷţ���ѡB��C�������ŷŵ�β����Ϊ��߿��ŷţ���Ⱦ������������̳������ŷ��ڿ����У���C���������⣻D��ֲ�����֣�ֲ����Ծ�����������ѡD��

��ϰ��ϵ�д�
�����Ŀ