��Ŀ����
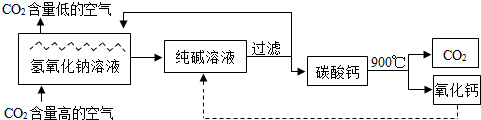
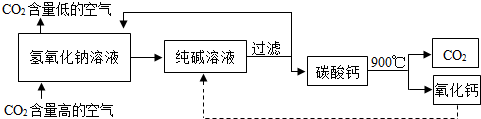
������̼�ġ������롰��桱��ʵ������������ŵ���Ҫ;��֮һ��ʵ�������У�������������NaOH��Һ��������CO2������ͼ��ͼ��ʾ����������������δ�������
�ٷ������н��еIJ����� ��
�ڸ�����ͼ������������У�����ˮ�ų������ȵ��������� ����ˮ������Ӧ�Ļ�ѧ����ʽΪ ��
�ۡ����ҡ��з����Ļ�ѧ��ӦΪ2NaOH+CO2�TNa2CO3+M����M�Ļ�ѧʽΪ �����������������У���ѭ��ʹ�õ������� ��

�ٷ������н��еIJ�����
�ڸ�����ͼ������������У�����ˮ�ų������ȵ���������
�ۡ����ҡ��з����Ļ�ѧ��ӦΪ2NaOH+CO2�TNa2CO3+M����M�Ļ�ѧʽΪ
��������1������̼���������ˮ���н��
��2�����������ƺ�ˮ��Ӧ�����������ƵĹ����зų������Ƚ��н��
��3�����ݶ�����̼������������Һ��Ӧ����̼���ƺ�ˮ���н��
��4������ѭ��ʾ��ͼ����ѭ��ʹ�õ����ʣ�
��2�����������ƺ�ˮ��Ӧ�����������ƵĹ����зų������Ƚ��н��
��3�����ݶ�����̼������������Һ��Ӧ����̼���ƺ�ˮ���н��
��4������ѭ��ʾ��ͼ����ѭ��ʹ�õ����ʣ�
����⣺��1��̼���������ˮ�����Է������н��еIJ����ǹ��ˣ�
��2�������ƺ�ˮ��Ӧ�����������ƵĹ����зų������ȣ���Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca��OH��2
��3��������̼������������Һ��Ӧ����̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪCO2+2NaOH=Na2CO3+H2O����M�Ļ�ѧʽΪH2O���ڴ˷�Ӧ�й����У������ƺ������������ظ����ã�
�ʴ�Ϊ����1������ ��2�������ƣ�CaO�� CaO+H2O=Ca��OH��2
��3��H2O CaO��NaOH �������ơ��������� ��
��2�������ƺ�ˮ��Ӧ�����������ƵĹ����зų������ȣ���Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca��OH��2
��3��������̼������������Һ��Ӧ����̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪCO2+2NaOH=Na2CO3+H2O����M�Ļ�ѧʽΪH2O���ڴ˷�Ӧ�й����У������ƺ������������ظ����ã�
�ʴ�Ϊ����1������ ��2�������ƣ�CaO�� CaO+H2O=Ca��OH��2
��3��H2O CaO��NaOH �������ơ��������� ��
�������������ⲻ��������ȫ������Ӷ���Ҳ�ǽ������п����ȵ����⣮��������Ҫ������������У�

��ϰ��ϵ�д�
�����Ŀ
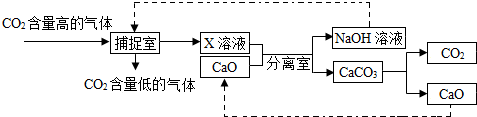
������̼�IJ�������ʵ������������ŵ���Ҫ;��֮һ����ѧ������NaOH��Һ���ܡ����������е�CO2������ͼ��������ڸ÷�����������ȷ���ǣ�������

| A���÷����а������ֽⷴӦ���ֽⷴӦ���û���Ӧ�� | B�����ʢ���Ca��OH��2��Һ | C���������ǹ��� | D����������������NaOH��CO2�������ʿ���ѭ������ |