��Ŀ����
��6�֣�ij��ѧС��������ͼ��ʾװ�òⶨͭп�Ͻ���Ʒ��п������������ͼ�й̶�װ������ȥ��̽���������£�
1������ʵ��װ�ò����װ��������
����B�м�������Ϊm��ͭп�Ͻ��ĩ����ע����A��B��������ϡ���ᣬ��B�в����������������ʱ���н����ɼУ������Ƴ�D�е��ܣ�ȷ��ȡ����¼��Ͳ��ˮ�������
�����˵Ȳ�����ȷ��������¼B��ʣ��������ʵ�������
IV����С��ļ�ͬѧ���ݷ�Ӧǰ��B�й������ʵ�����������Ʒ��п��������������ͬѧ�϶���Ͳ�ڲ��ˮ�����
��Ϊ��Ӧ�������������������ø������ڳ����µ��ܶȣ����ݻ�ѧ����ʽ������ؼ��㣬�õ���Ʒ��п��������������ش��������⣺
��1�����У���ȷ�IJ���˳���ǣ����ˡ� �� ��������
��2��ͨ������������ѧС�鷢����ͬѧ�������ݲ��ɿ�����ɸ����ݲ��ɿ���ԭ����Լ�������Ӱ���ǣ�
��II�У��Ƴ�D�еĵ�����������ˮ�����¼�����ƫС��
�� ��
��3�����ճ�������Ϊ����ʹ��ͨ���������ƳɺϽ�,��DZ�Ϊ��о��ͭ��һԪ��Ϊ��о������Ni���Ͻ����������Ӳ���õ��IJ��϶��� ����
��ѡ������Ӳ�ҵIJ��ϲ���Ҫ���ǵ������� ������ţ���
| A�������ĵ����� | B����������ʴ�� |
| C��������Ӳ�� | D�������۸���Ӳ����ֵ���Ǻ϶� |
Ni+ H2SO4=" Ni" SO4+ H2�� Fe+ Ni Cl 2=" Ni" + Fe Cl 2
���ֽ��������ǿ������˳��Ϊ ����Ԫ�ط��ű�ʾ����
��1��ϴ�� ��ɣ�������2�������ϡ������ų�һ���ֿ�����ʹ���ƫ��Ⱥ����𰸾��ɣ�
��3���ٽ��� ��A ��Al��Fe��Ni��Cu
���������������1��ʣ�������մ����Һ��Ӧ����ϴ�Ӻ�ɣ��Լ�������2������ϡ����Ҳռ�������Ҳ���ų�ˮ����3���ٺϽ������������Ʒ�����ڽ������ϣ�������Ӳ����Ҫ������ɱ�����Ӳ���ȶ��ԣ��͵������أ��۸��ݽ������˳��������Ԫ��ǰ��Ľ����ܺ��ᷴӦ�ų�����������ǰ��Ľ����ܰ����ں���Ľ��������ǵ�����Һ���û�������
���㣺���ý������ᷴӦ����װ��
������������Ŀ���ȵ����ͣ������Ϊ������������ʱҪ��ϸ���⣬ע��ǰ����ϵ������ϵ��ɺͻ���֪ʶ��

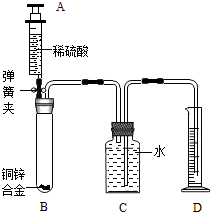 ��2012?�人��ij��ѧС��������ͼ��ʾװ�òⶨͭп�Ͻ���Ʒ��п������������ͼ�й̶�װ������ȥ��
��2012?�人��ij��ѧС��������ͼ��ʾװ�òⶨͭп�Ͻ���Ʒ��п������������ͼ�й̶�װ������ȥ��

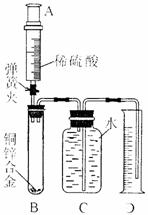 ��II�У��Ƴ�D�еĵ�����������ˮ�����¼�����ƫС��
��II�У��Ƴ�D�еĵ�����������ˮ�����¼�����ƫС��