��Ŀ����
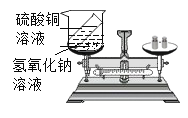
����Ŀ��ˮ������֮��Դ����������ĵ������70.8%��ˮ���ǡ��������ѧ��ѧ֪ʶ���:
��1����������Ӳ�ȹ����ˮ���������彡��,�����г���_______________________________����ˮ��Ӳˮ������ˮ�����ճ������г���_______________________________�ķ�������ˮ��Ӳ�ȡ�
��2������ˮ��������ɱ������,������ˮ������Ӧ�Ļ�ѧ����ʽΪ:![]() ,����X�Ļ�ѧʽ��__________________________________��
,����X�Ļ�ѧʽ��__________________________________��
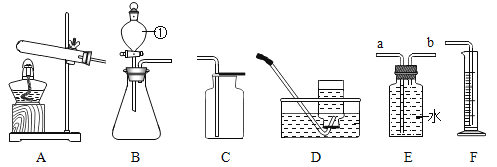
��3����ͼ��ʾ��ˮ��һЩ��ѧʵ���е���;,���з�����ȷ����_________��
A. 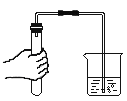 �ձ��е�ˮ���ڹ۲��Ƿ��������ų�
�ձ��е�ˮ���ڹ۲��Ƿ��������ų�
B.  ����ƿ�з�����ˮ���Է�ֹ����ƿը��
����ƿ�з�����ˮ���Է�ֹ����ƿը��
C.  ��ʵ��̽��ȼ�յ�������ˮֻ��������������á�
��ʵ��̽��ȼ�յ�������ˮֻ��������������á�
���𰸡�����ˮ ��� HCl AB .
��������
��1�������У�Ҫ����Ӳˮ����ˮ���ɼ������ˮ����Ӳˮ�����࣬��ĭ�٣���ˮ�и����٣���ĭ�࣬Ҫ����ˮ��Ӳ�ȣ��ɲ��ü�����еķ�����
�������ˮ ��С�
��2��![]() ���÷�Ӧ�У���Ӧǰ�У�2����ԭ�ӡ�2����ԭ�ӡ�1����ԭ�ӣ����������У�1����ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӣ�����X���У�1����ԭ�ӡ�1����ԭ�ӣ�X�Ļ�ѧʽ��HCl��
���÷�Ӧ�У���Ӧǰ�У�2����ԭ�ӡ�2����ԭ�ӡ�1����ԭ�ӣ����������У�1����ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӣ�����X���У�1����ԭ�ӡ�1����ԭ�ӣ�X�Ļ�ѧʽ��HCl��
���HCl��
��3��A�����װ�õ���������������ס�Թ������ܿ������ݲ�����װ�ò�©�����ձ��е�ˮ���ڹ۲��Ƿ��������ų�����ѡ��A��ȷ��
B����˿��������ȼ�գ�����ƿ�з�����ˮ���Է�ֹ��˿ȼ�����ɵĸ������ʽ�������������ƿ����ѡ��B��ȷ��
C��̽��ȼ�յ�������ˮ������������ṩ���������ã���ѡ��C����ȷ��
��ѡ��AB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ѧϰ�����Ļ�ѧ����ʱ����ʦ��ͬѧ������п��ϡ���ᷴӦ��ȡ������ʵ�顣��ͬѧ����ʦ��������п��Ũ���ᷴӦ����ȡ��������ʦ��ͬѧ����ȥ�����ϣ�Ȼ���п��Ũ���ᷴӦ�������������̽��
��������⣩п��Ũ������ȫ��Ӧ������������ʲô��
���������ϣ�
��1��Ũ������ǿ�������ᣬ���������������Mg��Zn��Cu�ȣ���Ӧ����SO2��
п��Ũ���ᷴӦ��![]()
��2��SO2��һ���д̼�����ζ���ж����壬��CO2�����ƵĻ�ѧ���ʣ���Ca��OH��2��Ӧ����������ˮ��CaSO3��������룩����1��ֻ��SO2 ����2��H2��SO2 ����3��_____��
��ʵ��̽����
ͬѧ������ʦָ������Ʋ���������ͼ��ʵ�飺
����������ۣ�
ʵ������ | ʵ����� |
��1��_____�� | ��SO2������ |
��2����ɫ������ͭ���ɫ | _____�� |
���ó����ۣ�����_____��ȷ��
����˼����չ��
��1��ʵ������������Ũ���ᡣŨ����2��������_____��
��2����ͬѧ�����ʵ����е���β��ܹ۲쵽��ɫ������ͭ���ɫ�������ʵ�鿪ʼ��CuO��û�����������ԭ��_____��
��3��ʵ�����ý�����Ũ���ᷴӦ��ȡ�ϴ���SO2���壬���н���Zn��Cu���ѡ�ý���_____������_____��
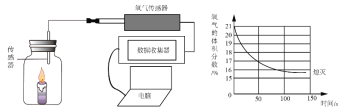
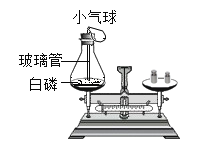
����Ŀ��ѧϰ�����غ㶨�ɺ�,ij��ѧ��ȤС���ͬѧ������ͼ��ʾ������ʵ��̽�������غ㶨�ɡ�
[�������]��ѧ��Ӧǰ������ʵ������ܺ��Ƿ����?
[�������]����1:�����;����2:��ȡ�
[ʵ��̽��]�ס��ҡ�������ͬѧ��������ƽ�ֱ������Ӧǰ�����ʵ�������
ʵ�鷽�� | ʵ������ | ���� | |
���� |
| �����������ݣ���ƽָ������ƫת�� | ����ȡ� ���¼��鷽���� Ӧǰ����������ȵ�ԭ����____________�� |
���� |
| ������ɫ��������ƽָ��_____________����������ƫת���� ������ƫת������������ƫת���� | ��� |
���� |
| _____________�� ��ƽָ��û�з���ƫת�� | ��� |
[��˼����]��̽����ѧ��Ӧǰ������ʵ������ܺ��Ƿ����ʱ���������������ɻ�μӵķ�Ӧһ��Ҫ��_____�н��С�
[�ó�����]����ͬѧ��װ�ý��иĽ����ٴ�̽��,���ܵó�����2��ȷ�Ľ��ۡ���ͬѧ�ǽ�һ�����۷������֣���Ӧ��ϵ�еķ�Ӧ������δ�μӷ�Ӧ�����������ڷ�Ӧǰ�ֲ���,���յó�������:_____________________�ĸ����ʵ������ܺ͵��ڷ�Ӧ�����ɵĸ����ʵ������ܺ͡�
[�۽���]��ѧ��Ӧǰ��ԭ�ӵ�_______________________û�иı䣬ԭ�ӵ���Ŀû��������ԭ�ӵ�����û�б仯�����������غ㡣