��Ŀ����
��2012?Ϋ����2011��ŵ������ѧ����������ɫ�п�ѧ�Ҵ��ᰣ��?л���������Ա��������������壮ʹ������ϼ������˳����塢�Ǿ���֮��ĵ���λ��Ա--���壮���й��ڲ��ϵ�˵����ȷ���ǣ�������
������A�����岻һ�����ǵ��ʣ�
B�������Ļ��������DZ���������Դ����Ҫ;����
C���������ڳ���������������Ӧ��
D�����칬һ�š�������������ǽ��������ϲ��ϣ�
B�������Ļ��������DZ���������Դ����Ҫ;����
C���������ڳ���������������Ӧ��
D�����칬һ�š�������������ǽ��������ϲ��ϣ�
����⣺A�����岻һ�����ǵ��ʣ���˵������
B�������Ļ��������DZ���������Դ����Ҫ;������˵����ȷ��
C���������ڳ���������������Ӧ���������ܵ������ﱡĤ���������ã����������кܺõĿ���ʴ���ܣ���˵������
D�����칬һ�š�������������ǽ��������ϲ��ϣ���˵������
��ѡB
B�������Ļ��������DZ���������Դ����Ҫ;������˵����ȷ��
C���������ڳ���������������Ӧ���������ܵ������ﱡĤ���������ã����������кܺõĿ���ʴ���ܣ���˵������
D�����칬һ�š�������������ǽ��������ϲ��ϣ���˵������
��ѡB
������������Դ����Ҫ����Դ֮һ���úñ��������ÿ���ʹ���ַ������ã�

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ
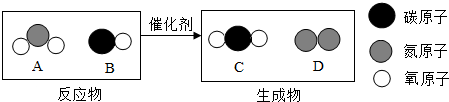
 ��2012?Ϋ�������и������ʼ�ͨ��һ����Ӧ����ʵ����ͼת������ס��ҡ������ܵ�����ǣ�������
��2012?Ϋ�������и������ʼ�ͨ��һ����Ӧ����ʵ����ͼת������ס��ҡ������ܵ�����ǣ�������