��Ŀ����
��1�������������������벻���������ϣ���ش��������⣺
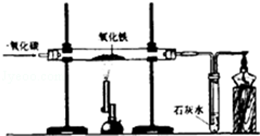
�ٳ�����øֲ����죬�������������ۣ����ÿ���Ч��ֹ�� �Ӵ������⣬��ҵ����һ����̼��ԭ������ұ���������Ļ�ѧ����ʽΪ
��ͭ���ڳ�ʪ�Ŀ����������⣬��ɷ�ΪCu2��OH��2CO3����������������ȥͭ���������
�۷Ͼɽ�����Ʒ��Ҫ���ⶪ����Ӧ�������ã��������������� ��д��һ����
��2����Դ�������������������ᷢչ������أ�
��PM2.5��ָ������ֱ��������2.5�Ŀ���������������������Ԫ��֮һ����д��һ���ܼ���PM2.5��Ⱦ�Ĵ�ʩ
�ڿ��Ʒ�Ӧ������ʹȼ�ϳ��ȼ�գ��Ӷ���Լ��Դ��ȼú����ʱ����ú������ú�ۣ�����Ƶ�������
(1) ��ˮ������; Fe2O3+3CO����2Fe+3CO2 ��ʳ��; �۽�Լ������Դ;(2) �ټ��ٻ�ʯȼ�ϵ�ʹ��;������ȼ��������ĽӴ����
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�������Դ�Dz�����������Դ,����������Դ�����ǿ̲��ݻ���ְ�������й���������ȷ����
| A���������ɿ����Ա��Ͻ������ϵĹ��� |
| B����߷Ͼɽ����Ļ��������� |
| C���ڽ�����Ʒ��ˢ�ᡢͿ�͵ȷ�ֹ������ʴ |
| D�����������ϴ����������������㲿�� |
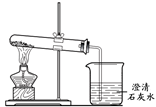
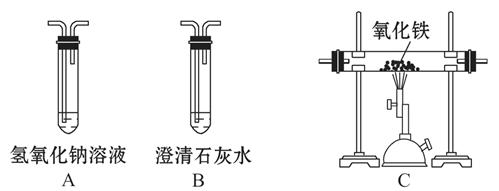
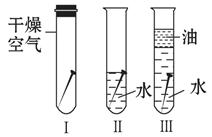
���ڳ�ʪ�Ŀ�����ᷢ����ʴ��֤������һ���μ��˷�Ӧ����Ҫ����ʵ����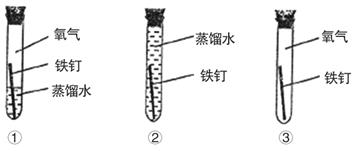
| A���٢ڡ����� | B���٢� | C���ڢ� | D���٢ڢ� |