��Ŀ����
������в��ϻش����⣺����һ 1703�꣬�¹���ѧ��ʩ����ϵͳ���ȼ��ѧ˵����Ϊ���ʿ���ȼ������Ϊ���Ǻ���ȼ�أ���ľ̿�ͽ���ȼ���ͷų���ȼ�ء���ʣ��ʧȥ��ȼ�ء������ң����ǣ�����ʼ��û���ҵ���ȼ�ء���
���϶� 1774�꣬������ѧ���������������������о�����ȼ���е����ã����������ѧ˵����Ϊȼ��������������������������ⷢ�ȵľ��ҷ�Ӧ�������ҿ���ȼ�յ�������ɴ��
��1������һ�У���������������ȼ�պ�ʧȥ��ȼ�ء��ġ����ҡ��ǣ��ѧʽ��______��
��2��ȼ�������ǡ�����ѧ˵������Ҫ���ݣ��谲�������������˶��ֿ���ȼ�յĴ�ʩ��
��ȷ�����õ�ͨ�磬���ܱ�֤�˵İ�ȫ�⣬����ʹͨ�������ij������ͳ��ȼ�գ����ǿ��ǵ����ȼ����Ҫ______��
������ʹ��������������ȼ���ϣ���������ʱ�ֽ��ˮ���������µ�����������ѧ����ʽΪ______ Al2O3+3H2O
���𰸡���������1������������ȼ�գ�����������������
��2���ٸ�������ȼ�յ�����������ȼ�յ������ǣ���1�����ʾ��п�ȼ�ԣ���2����ȼ���������Ӵ�����3���¶ȴﵽ��ȼ����Ż�㣮�ڸ�����Ϣ��������������ʱ�ֽ��ˮ���������µ���������д����ѧ��Ӧʽ��
��3��������Ŀ�ṩ����Ϣ�Ͷ�ȼ��֪ʶ���˽�������⣮
����⣺��1����������������ȼ�պ�ʧȥ��ȼ�ء��ġ����ҡ�����������������ѧʽ��Fe3O4��
�ʴ�Ϊ��Fe3O4��
��2����ȷ�����õ�ͨ�磬���ܱ�֤�˵İ�ȫ�⣬����ʹͨ�������ij������ͳ��ȼ�գ����ǿ��ǵ����ȼ����Ҫ�����������
�ʴ�Ϊ�������������
��������������ʱ�ֽ��ˮ���������µ�����������ѧ����ʽΪ��2Al��OH��3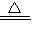 Al2O3+3H2O��
Al2O3+3H2O��
�ʴ�Ϊ��2Al��OH��3 Al2O3+3H2O��
Al2O3+3H2O��
��3��1��CO�ĺ���С��12.5%ʱ���ڿ����дﵽ�Ż��Ҳ����ȼ�գ�˵����ȼ��ȼ����Ҫ�ﵽһ����Ũ�ȣ������ȼ��ȼ����Ҫ�ﵽһ����Ũ�ȣ�
2��ľ̿������Ũ�ȵ���14%�Ŀ����У��ﵽ�Ż��Ҳ����ȼ�գ�˵��ֻ�������ﵽһ��Ũ��ʱ����ȼ�����ȼ�գ����ֻ�������ﵽһ��Ũ��ʱ����ȼ�����ȼ�գ�
3���ƿ���������ȼ�գ�þ���ڶ�����̼��ȼ�գ�˵��ȼ�ղ�һ����Ҫ���������ȼ�ղ�һ����Ҫ������
������������Ҫ����ȼ�����������֪ʶ�����ʱҪ����ȼ�յ�������������ͬʱ�߱���ȱһ���ɣ�����ͨ����3����Ҫ��ͬѧ���ܹ�������Ϣ���⣮
��2���ٸ�������ȼ�յ�����������ȼ�յ������ǣ���1�����ʾ��п�ȼ�ԣ���2����ȼ���������Ӵ�����3���¶ȴﵽ��ȼ����Ż�㣮�ڸ�����Ϣ��������������ʱ�ֽ��ˮ���������µ���������д����ѧ��Ӧʽ��
��3��������Ŀ�ṩ����Ϣ�Ͷ�ȼ��֪ʶ���˽�������⣮
����⣺��1����������������ȼ�պ�ʧȥ��ȼ�ء��ġ����ҡ�����������������ѧʽ��Fe3O4��
�ʴ�Ϊ��Fe3O4��
��2����ȷ�����õ�ͨ�磬���ܱ�֤�˵İ�ȫ�⣬����ʹͨ�������ij������ͳ��ȼ�գ����ǿ��ǵ����ȼ����Ҫ�����������
�ʴ�Ϊ�������������
��������������ʱ�ֽ��ˮ���������µ�����������ѧ����ʽΪ��2Al��OH��3
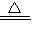 Al2O3+3H2O��
Al2O3+3H2O���ʴ�Ϊ��2Al��OH��3
 Al2O3+3H2O��
Al2O3+3H2O����3��1��CO�ĺ���С��12.5%ʱ���ڿ����дﵽ�Ż��Ҳ����ȼ�գ�˵����ȼ��ȼ����Ҫ�ﵽһ����Ũ�ȣ������ȼ��ȼ����Ҫ�ﵽһ����Ũ�ȣ�
2��ľ̿������Ũ�ȵ���14%�Ŀ����У��ﵽ�Ż��Ҳ����ȼ�գ�˵��ֻ�������ﵽһ��Ũ��ʱ����ȼ�����ȼ�գ����ֻ�������ﵽһ��Ũ��ʱ����ȼ�����ȼ�գ�
3���ƿ���������ȼ�գ�þ���ڶ�����̼��ȼ�գ�˵��ȼ�ղ�һ����Ҫ���������ȼ�ղ�һ����Ҫ������
������������Ҫ����ȼ�����������֪ʶ�����ʱҪ����ȼ�յ�������������ͬʱ�߱���ȱһ���ɣ�����ͨ����3����Ҫ��ͬѧ���ܹ�������Ϣ���⣮

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
 ��2006?��ƽ���Ķ����в��ϣ���ϻ�ѧ֪ʶ�ش����⣮
��2006?��ƽ���Ķ����в��ϣ���ϻ�ѧ֪ʶ�ش����⣮