��Ŀ����
ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��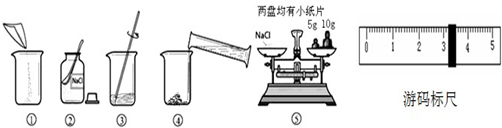
��1������ͼ��ʾ����ű�ʾ������Һ����ȷ����˳��______��
��2��ͼ���У���һ��������������������______��
��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ������ͼ�����ȡ��NaCl����Ϊ______��
��4�����ݼ�����Ҫ��ȡˮ�������______��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����______����ѡ����ĸ��ţ�

��5������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ��������������______������ڡ�����С�ڡ����ڡ���10%��
��6���������Һ��������������10%������������ܵ�ԭ��д��������______��______��

��1������ͼ��ʾ����ű�ʾ������Һ����ȷ����˳��______��
��2��ͼ���У���һ��������������������______��
��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ������ͼ�����ȡ��NaCl����Ϊ______��
��4�����ݼ�����Ҫ��ȡˮ�������______��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����______����ѡ����ĸ��ţ�

��5������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ��������������______������ڡ�����С�ڡ����ڡ���10%��
��6���������Һ��������������10%������������ܵ�ԭ��д��������______��______��
��1������ʳ�ε��������ȴ��ƿ���������������ϣ���ȡ�õ�ʳ�η�����ƽ�����̣�Ȼ�Ƶõ�ʳ�ηŵ��ձ��У��ٽ���ȡ��ˮ�����ձ��У��ò���������ʹ֮�ܽ⣬����ȷ��˳��Ϊ�ڢݢ٢ܢۣ�
��2��ͼ���е���������Ϊҩ�ף�
��3��ʳ�ε�����=����+���룬��ͼ��֪������Ķ�����15g������Ķ�����3.2g����ʳ�ε�����=15g+3.2g=18.2g��
��4���Ȼ�����Һ������=18.2g��10%=182g������ˮ������=182g-18.2g=163.8g��������ȡˮ�������163.8ml����ȡ����ʱ������Ӧ��Һ�尼Һ�����ʹ�����ˮƽ����ѡD��
��5������ȱ��һ���������ʳ�ε�������С�����ʼ��٣�����Һ��ϡ����������������С��
��6��������Һ�����Һ��������������10%��������������ƫ���ԭ�������Ȼ�����ƫ����ܼ�ˮ����ƫС��
�ʴ𣺹ʴ�Ϊ����1���ڢݢ٢ܢۣ�
��2��ҩ�ף�
��3��18.2g��
��4��163.8ml��D��
��5��С�ڣ�
��6������NaCl��ʵ�������ȼ�����������ˮ��ʵ�����ȼ�����С��
��2��ͼ���е���������Ϊҩ�ף�
��3��ʳ�ε�����=����+���룬��ͼ��֪������Ķ�����15g������Ķ�����3.2g����ʳ�ε�����=15g+3.2g=18.2g��
��4���Ȼ�����Һ������=18.2g��10%=182g������ˮ������=182g-18.2g=163.8g��������ȡˮ�������163.8ml����ȡ����ʱ������Ӧ��Һ�尼Һ�����ʹ�����ˮƽ����ѡD��
��5������ȱ��һ���������ʳ�ε�������С�����ʼ��٣�����Һ��ϡ����������������С��
��6��������Һ�����Һ��������������10%��������������ƫ���ԭ�������Ȼ�����ƫ����ܼ�ˮ����ƫС��
�ʴ𣺹ʴ�Ϊ����1���ڢݢ٢ܢۣ�
��2��ҩ�ף�
��3��18.2g��
��4��163.8ml��D��
��5��С�ڣ�
��6������NaCl��ʵ�������ȼ�����������ˮ��ʵ�����ȼ�����С��

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ


 ΪԪ�����ڱ����й�̼Ԫ�ص���Ϣ��̼Ԫ��ԭ�Ӻ��ڵ�������Ϊ�� ����
ΪԪ�����ڱ����й�̼Ԫ�ص���Ϣ��̼Ԫ��ԭ�Ӻ��ڵ�������Ϊ�� ����
