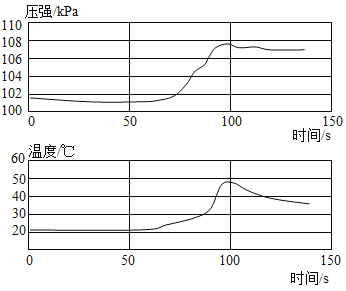
��Ŀ����
�ҹ�ú̿��Դ�ḻ��Ŀǰ���dz��˽�ú��Ϊȼ���⣬����עú������������ҵ����ú�Ϳ���Ϊԭ����������[CO(NH2)2]��һ���������£�
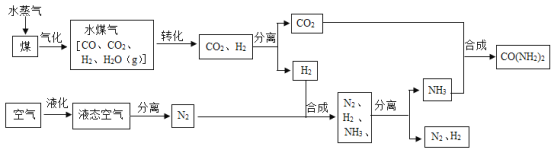
��1����Һ̬�����з����N2�Ĺ�������_____�����������ѧ�����仯��
��2����ú��ˮ������Ӧǰ���Ƚ�ú���飬��������Ŀ����______��
��3��ˮú����ͭ����ʵ��CO��ת����CO+H2O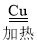 CO2+X������X�Ļ�ѧʽΪ____��
CO2+X������X�Ļ�ѧʽΪ____��
��4�����������У���һ�������ºϳ����ص�ͬʱ����ˮ���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ____��
��5��ʵ�������У���Ӧ��������ȫ���С����������п���ѭ�����õ�������______��
��6���ϳɵ�����[CO(NH2)2]����Ҫ�Ļ�ѧ���ϣ����������������[ (NH4)2 SO4]�ɽ������²������ֱ�ȡ�����Թ��У���������������Һ�����ȣ����Թܿڷ�һ��ʪ���______ɫʯ����ֽ���۲���ɫ�仯��
���ջ�ѧ����Ի�ѧ��ѧϰ������Ҫ��
��1��д���������ʵĻ�ѧʽ
����_______������_____________������____________������______
��2��������Һ�������д�±��� �����ʵĻ�ѧʽ��ʾ��
��Һ | ʯ��ˮ | ϡ���� | ��� | ������ˮ | ̼������Һ |
���� | ______ | ______ | ______ | ______ | ______ |
�ܼ� | ˮ | ______ | �ƾ� | ˮ | ˮ |
��3��þ�۱����������������û�ѧ����ʽ��ʾ��ԭ����_____________;
��4���г��ϳ��۵IJ�Ѫ��Ƭ�г�����������ϸС�Ļ�ԭ�����ۣ�����������θҺ�е����ᷴӦת��Ϊ�����������Ѫ�����ã�д�������Ӧ�Ļ�ѧ����ʽ:____________��
��5���������Ĵ������˴����ĸ�����д����һ����̼�ڸ����»�ԭ��������Ҫ�ɷ����������Ļ�ѧ����ʽ: ______________��
��6���谲����������������þ��Ϊ��ȼ����,�����ȷֽ�����ˮ���������µ�����þ��д���÷�Ӧ�Ļ�ѧ����ʽ: _____________��