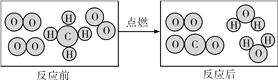
��Ŀ����
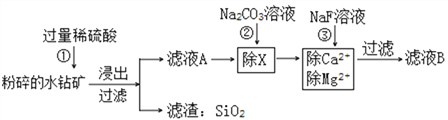
����Ŀ��ˮ����г�SiO2�⣬����CoO��Fe2O3��CaO��MgO����ˮ�������ȡ��(Co)��Ԥ�����������£�
(1)д��������з�����Ӧ��һ����ѧ����ʽ��_____________________��
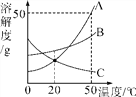
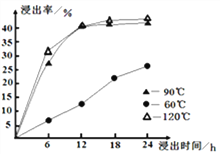
(2)�������һ��Ũ�ȵ�ϡ��������Ľ�������ʱ����¶ȵı仯����ͼ��ʾ�����������ɱ���Ч�ʣ���ѵĽ����¶���___________����ѵĽ���ʱ����____________��
(3)��ҺA�д�������______________�������ӣ�
(4)�����)��ȥ�Ľ���������Ҫ��______________��
(5)�������ͨ�����ֽⷴӦ��Ca2+��Mg2+ת����_______________(�ѧʽ)��������ȥ��
(6)��ҺB�д������ڵĽ���������Co2+��_____________(�ѧ����)��
���𰸡� MgO+H2SO4===MgSO4+H2O 90�� 12h 5 Fe3+ CaF2��MgF2 Na+
�������������ڴ�ˮ�������ȡ��(Co)��Ԥ�������龳�¿����˽�������������ķ�Ӧ�����ֽⷴӦ�������龳�л�ȡ��Ϣ��������
(1)�����������þ�����ᷴӦ��������þ��ˮ����Ӧ�Ļ�ѧ����ʽ��MgO+H2SO4===MgSO4+H2O��
��2������ͼʾ��֪��ѵĽ����¶���90�棬��ѵĽ���ʱ����12h����ʱ�����ʴ�40%��
(3) ˮ����е�CoO��Fe2O3��CaO��MgO����������ᷴӦ�ֱ������������ܡ�������������ƺ�����þ����ҺA�д�������Co2+��Fe3+��Ca2+��Mg2+��H+5�������ӣ�
(4)�������̿�֪������dz�ȥCa2+��Mg2+��Co2+��������ҺB�У�����ڳ�ȥ�Ľ���������Ҫ��Fe3+��
(5)�������ͨ�����ֽⷴӦ��Ca2+��Mg2+ת����CaF2��MgF2��������ȥ��
(6) �ڲ���ڢ��м����Na+������û�б����ġ���ҺB�д������ڵĽ���������Co2+��Na+��

����Ŀ������ͼ��ijƷ�Ƴ´IJ��ֱ�ǩ��

(1)ԭ����Ŵ�ס�����������Ҫ�ɷֶ���___________��
A.̼ˮ������ B.����֬ C.������
(2)ԭ���е�ʳ���ɹ���____________��
A.���� B.ԭ�� C.����
(3)�ó´��������������Ǵ��Ź�ʱ��д������(HAc)����������Ӧ�Ļ�ѧ����ʽ��___________
(4)��֪ʳ�ĵȼ����±����֣���ƿ�´ĵȼ�_____________(��ؼ����ż���һ��������)
�ȼ� | �ؼ� | �ż� | һ�� | ���� |
����g/100mL | ��6.00 | 5.50~5.99 | 5.00~5.49 | 4.50~4.99 |
(5)�������ѧ֪ʶ�ٲ���һ���ó´���������___________________________________��